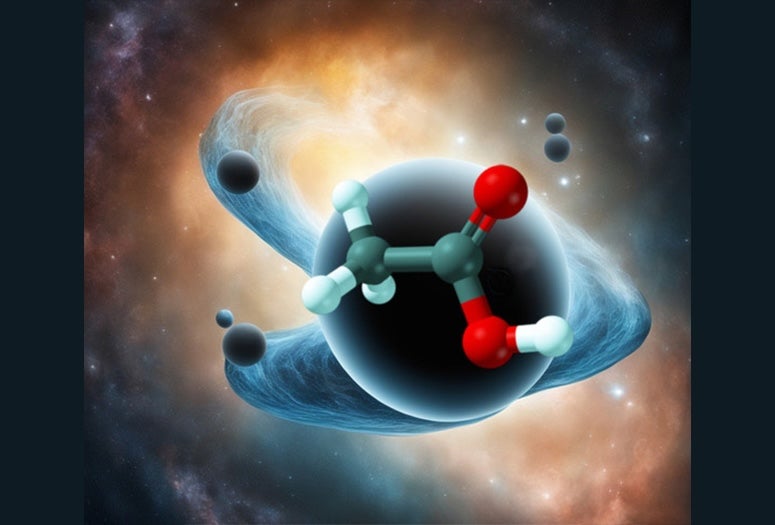
If you were to throw a message in a bottle into a black hole, all of the information in it, down to the quantum level, would become completely scrambled. Because in black holes this scrambling happens as quickly and thoroughly as quantum mechanics allows, they are generally considered nature’s ultimate information scramblers.
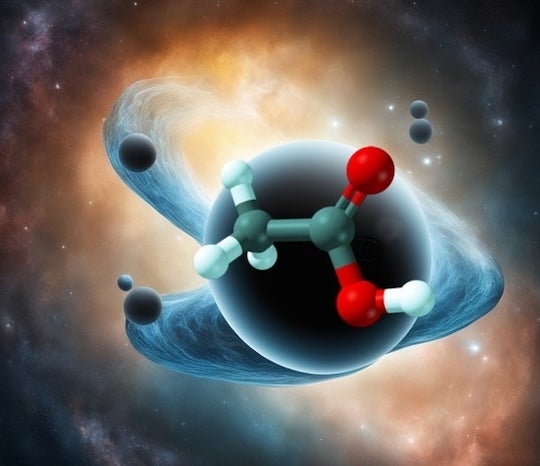
New research from Rice University theorist Peter Wolynes and collaborators at the University of Illinois Urbana-Champaign, however, shows that molecules can be as formidable at scrambling quantum information as black holes. Combining mathematical tools from black hole physics and chemical physics, they have shown that quantum information scrambling takes place in chemical reactions and can nearly reach the same quantum mechanical limit as it does in black holes. The work is published online in the Proceedings of the National Academy of Sciences.
“This study addresses a long-standing problem in chemical physics, which has to do with the question of how fast quantum information gets scrambled in molecules,” Wolynes said. “When people think about a reaction where two molecules come together, they think the atoms only perform a single motion where a bond is made or a bond is broken.
“But from the quantum mechanical point of view, even a very small molecule is a very complicated system. Much like the orbits in the solar system, a molecule has a huge number of possible styles of motion ⎯ things we call quantum states. When a chemical reaction takes place, quantum information about the quantum states of the reactants becomes scrambled, and we want to know how information scrambling affects the reaction rate.”
To better understand how quantum information is scrambled in chemical reactions, the scientists borrowed a mathematical tool typically used in black hole physics known as out-of-time-order correlators, or OTOCs.
“OTOCs were actually invented in a very different context about 55 years ago, when they were used to look at how electrons in superconductors are affected by disturbances from an impurity,” Wolynes said. “They’re a very specialized object that is used in the theory of superconductivity. They were next used by physicists in the 1990s studying black holes and string theory.”

OTOCs measure how much tweaking one part of a quantum system at some instant in time will affect the motions of the other parts ⎯ providing insight into how quickly and effectively information can spread throughout the molecule. They are the quantum analog of Lyapunov exponents, which measure unpredictability in classical chaotic systems.
“How quickly an OTOC increases with time tells you how quickly information is being scrambled in the quantum system, meaning how many more random looking states are getting accessed,” said Martin Gruebele, a chemist at Illinois Urbana-Champaign and co-author on the study who is a part of the joint Rice-Illinois Center for Adapting Flaws as Features funded by the National Science Foundation. “Chemists are very conflicted about scrambling in chemical reactions, because scrambling is necessary to get to the reaction goal, but it also messes up your control over the reaction.
“Understanding under what circumstances molecules scramble information and under what circumstances they don’t potentially gives us a handle on actually being able to control the reactions better. Knowing OTOCs basically allows us to set limits on when this information is really disappearing out of our control and conversely when we could still harness it to have controlled outcomes.”
In classical mechanics, a particle must have enough energy to overcome an energy barrier for a reaction to occur. However, in quantum mechanics, there’s the possibility that particles can “tunnel” through this barrier even if they don’t possess sufficient energy. The calculation of OTOCs showed that chemical reactions with a low activation energy at low temperatures where tunneling dominates can scramble information at nearly the quantum limit, like a black hole.
Nancy Makri, also a chemist at Illinois Urbana-Champaign, used path integral methods she has developed to study what happens when the simple chemical reaction model is embedded in a larger system, which could be a large molecule’s own vibrations or a solvent, and tends to suppress chaotic motion.

“In a separate study, we found that large environments tend to make things more regular and suppress the effects that we’re talking about,” Makri said. “So we calculated the OTOC for a tunneling system interacting with a large environment, and what we saw was that the scrambling was quenched ⎯ a big change in the behavior.”
One area of practical application for the research findings is to place limits on how tunneling systems can be used to build qubits for quantum computers. One needs to minimize information scrambling between interacting tunneling systems to improve the reliability of quantum computers. The research could also be relevant for light-driven reactions and advanced materials design.
“There’s potential for extending these ideas to processes where you wouldn’t just be tunneling in one particular reaction, but where you’d have multiple tunneling steps, because that’s what’s involved in, for example, electron conduction in a lot of the new soft quantum materials like perovskites that are being used to make solar cells and things like that,” Gruebele said.
Wolynes is Rice’s D.R. Bullard-Welch Foundation Professor of Science, a professor of chemistry, f biochemistry and cell biology, physics and astronomy and materials science and nanoengineering and co-director of its Center for Theoretical Biological Physics, which is funded by the National Science Foundation. Co-authors Gruebele is the James R. Eiszner Endowed Chair in Chemistry; Makri is the Edward William and Jane Marr Gutgsell Professor and professor of chemistry and physics; Chenghao Zhang was a graduate student in physics at Illinois Urbana-Champaign and is now a postdoc at Pacific Northwest National Lab; and Sohang Kundu recently received his Ph.D. in chemistry from the University of Illinois and is currently a postdoc at Columbia University.
The research was supported by the National Science Foundation (1548562, 2019745, 1955302) and the Bullard-Welch Chair at Rice (C-0016).
- Peer-reviewed paper: “Quantum information scrambling and chemical reactions” | Proceedings of the National Academy of Sciences | DOI: 10.1073/pnas.2321668121
Authors: Chenghao Zhang, Sohang Kundu, Nancy Makri, Martin Gruebele and Peter Wolynes
https://doi.org/10.1073/pnas.2321668121 - Image downloads:
https://news-network.rice.edu/news/files/2024/04/BlackHoleMolecule2-0387e2be407aefaa.jpg
CAPTION: Rice University theorist Peter Wolynes and collaborators at the University of Illinois Urbana-Champaign have shown that molecules can be as formidable at scrambling quantum information as black holes. (Image courtesy of Martin Gruebele; DeepAI was used in image production)
https://news-network.rice.edu/news/files/2024/04/Wolynes_Makri_Gruebele-3e4286b5ec5ad9fc.jpg
CAPTION: Peter Wolynes (from left), Nancy Makri and Martin Gruebele (Photo of Wolynes by Gustavo Raskosky/Rice University; photo of Makri courtesy of Nancy Makri; photo of Gruebele by Fred Zwicky/University of Illinois Urbana-Champaign)
https://news-network.rice.edu/news/files/2024/04/Zhang_Kundu-66fa4d09a6f863a9.jpg
CAPTION: Chenghao Zhang (left) and Sohang Kundu (Photo of Zhang by Bill Wiegand/University of Illinois Urbana-Champaign; photo of Kundu courtesy of Sohang Kundu)
- Related stories:
-
Theory can sort order from chaos in complex quantum systems:
https://news.rice.edu/news/2023/theory-can-sort-order-chaos-complex-quantum-systems
Rice lab’s quantum simulator delivers new insight:
https://news.rice.edu/news/2022/rice-labs-quantum-simulator-delivers-new-insight
NSF renews Rice biological physics center:
https://news.rice.edu/news/2020/nsf-renews-rice-biological-physics-center - Links:
-
Center for Adapting Flaws as Features: https://nsfcaff.org/
Gruebele Lab: https://gruebele-group.chemistry.illinois.edu/
BioScience Research Collaborative: https://brc.rice.edu/
Center for Theoretical Biological Physics: https://ctbp.rice.edu/
Department of Chemical and Biomolecular Engineering: https://chbe.rice.edu/
Department of Chemistry: https://chemistry.rice.edu/
Department of Physics and Astronomy: https://physics.rice.edu/
George R. Brown School of Engineering: https://engineering.rice.edu
Ken Kennedy Institute: https://kenkennedy.rice.edu/
Wiess School of Natural Sciences: https://naturalsciences.rice.edu
Wolynes Research Lab: https://wolynes.rice.edu/
Makri Group: http://makri.scs.illinois.edu/
Department of Chemistry, University of Illinois: https://chemistry.illinois.edu/