That stubborn athlete’s foot infection an estimated 70% of people get at some point in their life could become much easier to get rid of thanks to nanoscale drills activated by visible light.
Proven effective against antibiotic-resistant infectious bacteria and cancer cells, the molecular machines developed by Rice University chemist James Tour and collaborators are just as good at combating infectious fungi, according to a new study published in Advanced Science.
Based on the work of Nobel laureate Bernard Feringa, the Tour group’s molecular machines are nanoscale compounds whose paddlelike chain of atoms moves in a single direction when exposed to visible light. This causes a drilling motion that allows the machines to bore into the surface of cells, killing them.
“Dr. Tour posed the question of whether they can also kill fungi, which had never been explored before,” said lead co-author Ana Santos, a Rice alumna who is currently a Marie Curie Global Postdoctoral Fellow at Fundación Instituto de Investigación Sanitaria Islas Baleares in Spain. “Our study is the first to show that, indeed, these molecules can also be effective against fungi."
Fungal infections pose a particular threat to patients with a weakened immune system, such as cancer patients and transplant recipients. The cost of treating bacterial infections in the U.S. alone is estimated at more than $7 billion per year.
COVID-19 has made matters worse. Immunosuppressants were widely used early in the pandemic to reduce the risk of long-term organ damage caused by an overactive immune system in response to the virus, a tactic that allowed fungal infections to proliferate.
“In the aftermath of that first wave of the pandemic, doctors started seeing an increase in cases of mucormycosis, or ‘black fungus,’ a normally rare fungal infection which causes a pneumonia-like illness, as a result of the overuse of immunosuppressant drugs,” Santos said. “We want to develop a way to combat fungal infections that does not tax a weakened immune system further, and we hope these molecular machines might be a way to do so.”
Santos said overuse of antifungals in agriculture is also contributing to resistance in humans.
“This is an emergent phenomenon that we are just starting to understand,” she said. “Antifungals are used in agriculture to combat damage to crops caused by fungal infestation. However, most of the antifungal drugs that are used in agriculture are also used in humans. Therefore, overuse of antifungals can lead to resistance not just in the fungi that cause plant illnesses but also in other fungi, including those that can be harmful to humans.”
In contrast to most antifungals, development of resistance to the visible-light activated nanoscale drills was not detected. Spinning at 2-3 million times per second, their rotors cause fungal cells to disintegrate by disrupting their metabolism.

“There are only a few classes of antifungals in clinical use,” Santos said. “These conventional antifungals typically employ one of a few different mechanisms of action, including inhibiting the synthesis of the fungal cell wall, targeting the fungal cell membrane or inhibiting the production of ergosterol, which is an essential component for normal fungal cell membrane structure.
“Our molecules differ from conventional antifungals in that they specifically target what we call the powerhouses of the cell, that is, the mitochondria,” she continued. Mitochondria are responsible for producing adenosine triphosphate, or ATP, which drives cellular metabolism.
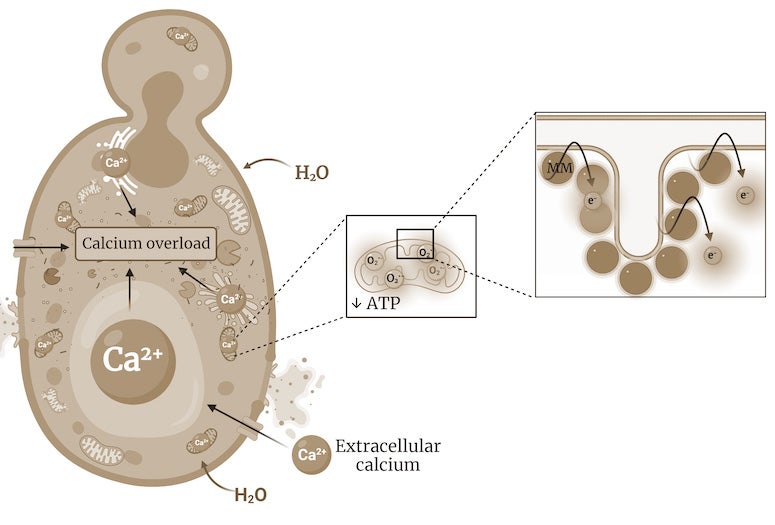
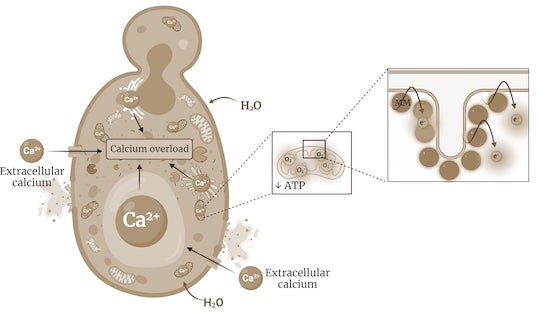
"By targeting the mitochondria, our molecules disrupt the cell's metabolism, resulting in an overall energy imbalance that leads to an uncontrolled flow of water and ions such as calcium into the cell, eventually causing the cell to explode," Santos explained.
Tour is the T. T. and W. F. Chao Professor of Chemistry and a professor of materials science and nanoengineering. Rice graduate student Jacob L. Beckham is a lead co-author on the study along with Santos.
The European Union’s Horizon 2020 program (843116), the National Science Foundation Graduate Research Fellowship Program, the Discovery Institute, the Robert A. Welch Foundation (C-2017-20190330) and the DEVCOM Army Research Laboratory (W911NF-19-2-0269, W911NF-18-2-0234) supported the research.
-30-
- Peer-reviewed paper:
-
Visible-light-activated molecular machines kill fungi by necrosis following mitochondrial dysfunction and calcium overload | Advanced Science | DOI: 10.1002/advs.202205781
https://doi.org/10.1002/advs.202205781
Authors: Ana Santos, Jacob Beckham, Dongdong Liu, Gang Li, Alexis van Venrooy, Antonio Oliver, George Tegos and James Tour - Image downloads:
-
https://news-network.rice.edu/news/files/2023/01/Tour_fitlow_LG.jpg
CAPTION: James Tour is the T. T. and W. F. Chao Professor of Chemistry and a professor of materials science and nanoengineering. (Photo by Jeff Fitlow/Rice University)https://news-network.rice.edu/news/files/2023/01/Schematic-MOA-Antifungal_LG.jpg
CAPTION: Schematic representation of the mechanisms by which light-activated molecular machines kill fungi. Molecular machines bind to fungal mitochondria, decreasing adenosine triphosphate (ATP) production and impairing the function of energy-dependent transporters that control the movement of ions, such as calcium. This leads to the influx of water, which causes the organelles to swell and eventually the cells to burst. (Image courtesy of Tour Group/Rice University)https://news-network.rice.edu/news/files/2023/01/Electron-microscopy-photo_LG.jpg
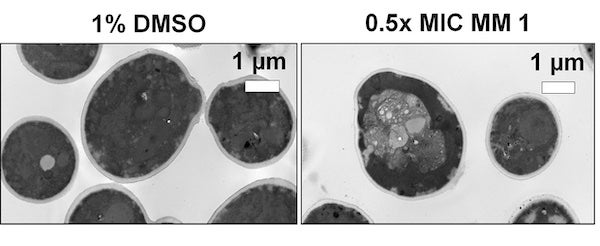
CAPTION: Ultrastructural changes induced by light-activated molecular machines in the fungus Candida albicans, detected by transmission electron microscopy, compared to a solvent control (1% dimethyl sulfoxide). (Image courtesy of Matthew Meyer, Electron Microscopy Facilities/Rice University)
- Related stories:
-
New weapon targets antibiotic resistance:
https://news.rice.edu/news/2022/new-weapon-targets-antibiotic-resistance
Bacteria-killing drills get an upgrade:
https://news.rice.edu/news/2022/bacteria-killing-drills-get-upgrade
Chemists build a better cancer-killing drill:
https://news2.rice.edu/2019/05/28/chemists-build-a-better-cancer-killing-drill-2/
Deadly ‘superbugs’ destroyed by molecular drills:
https://news2.rice.edu/2019/12/12/deadly-superbugs-destroyed-by-molecular-drills/
Motorized molecules drill through cells:
https://news2.rice.edu/2017/08/30/motorized-molecules-drill-through-cells-2/ - Links:
-
Tour Group: https://www.jmtour.com/
Rice Department of Chemistry: https://chemistry.rice.edu/
Wiess School of Natural Sciences: https://naturalsciences.rice.edu/
- About Rice:
-
Located on a 300-acre forested campus in Houston, Rice University is consistently ranked among the nation’s top 20 universities by U.S. News & World Report. Rice has highly respected schools of Architecture, Business, Continuing Studies, Engineering, Humanities, Music, Natural Sciences and Social Sciences and is home to the Baker Institute for Public Policy. With 4,240 undergraduates and 3,972 graduate students, Rice’s undergraduate student-to-faculty ratio is just under 6-to-1. Its residential college system builds close-knit communities and lifelong friendships, just one reason why Rice is ranked No. 1 for lots of race/class interaction and No. 1 for quality of life by the Princeton Review. Rice is also rated as a best value among private universities by Kiplinger’s Personal Finance.