Demand for valuable metals needed in batteries is poised to grow over the coming decades in step with the growth of clean energy technologies, and the best place to source them may be by recycling spent batteries.

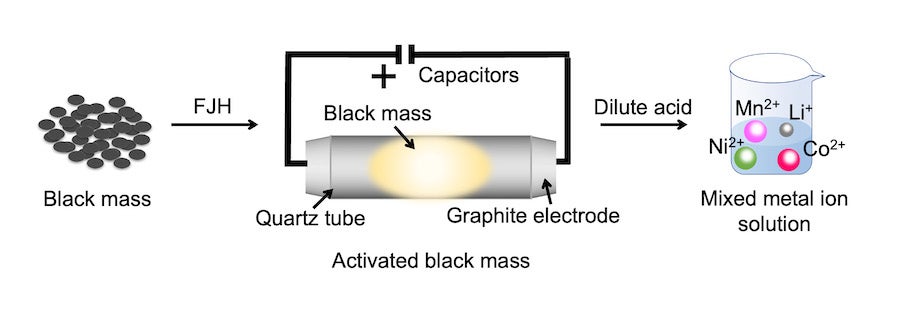
A battery recycling process developed by Rice University scientists can remove the inert layer on battery metals and lower their oxidation state, making them soluble in low-concentration acid. Using its signature Joule-heating technique to bring the combined cathode and anode waste to temperatures above 2100 degrees Kelvin in seconds, the lab of Rice chemist James Tour achieved a metal recovery yield exceeding 98% from various types of mixed battery waste.
“We developed a high-yield, low-cost method of reclaiming metals directly from ‘black mass’ ⎯ the combined cathode and anode waste the industry traditionally tries to recycle ⎯ that significantly reduces the environmental footprint of spent battery processing,” said Jinhang Chen, a Rice chemistry graduate student and co-lead author on a study published in Science Advances.
Not only does the new method significantly reduce secondary waste streams from the contaminated, acidic leaching solutions, but it also cuts the duration of the recycling process by almost 100-fold.
“It takes less than 20 minutes to dissolve the same amounts as opposed to 24 hours,” Jinhang Chen said. “Our findings have the potential to reduce the cost of battery waste recycling through decreased energy, water and acid consumption and lower carbon dioxide emissions.
“Notably, the metals’ leaching kinetics is improved from the combined effects of decomposing the passivated layer and regulating the metal valence state for the first time,” said Weiyin Chen, a former Rice chemistry graduate student and co-lead author.
The process could help supercharge the battery recycling business ⎯ a market that’s expected to grow rapidly as the batteries powering electrical vehicles and other electronics expire.
“Battery recycling is a very big deal, especially now,” said Tour, Rice’s T.T. and W.F. Chao Professor of Chemistry and a professor of materials science and nanoengineering. “Batteries in electric vehicles last about 10 years, and many of those are coming due now, because it’s been about 10 years that we’ve been using them.”
(Image courtesy of the Tour lab/Rice University)
Aside from helping reduce the environmental harms of mining, recycling spent batteries is an economically sound practice as the concentration of metals like cobalt and nickel is higher in many types of lithium-ion batteries than in natural ores.
“Currently, 95% of batteries are not recycled because we don’t have the capacity to recycle them, even as waste from electronics is increasing at an annual rate of 9%” said Tour, whose lab previously developed a process for recycling battery anodes. “A lot of current battery recycling processes involve the use of very strong acids, and these tend to be messy, cumbersome processes.
“What we found is that if you ‘flash’ the black mass, then you can easily separate out the critical metals using only low-concentration hydrochloric acid. You could say the flash liberates the metals, so they dissolve easier. We’re still using acid, but much less. That’s why the economics is so much better.”
The study includes a life-cycle assessment comparing the new process to different current methods of battery recycling.
“This study has the capacity to motivate the growth of battery waste management and contribute to the mass production of electrical vehicles at a more competitive cost by lowering the cost of battery production,” Chen said.
The research was supported by the Air Force Office of Scientific Research (FA9550-22-1-0526), the U.S. Army Engineer Research and Development Center (W912HZ-21-2-0050), the Department of Energy’s Basic Energy Sciences program (DE-SC0012547) and the Welch Foundation (C-2065-20210327).
- Peer-reviewed paper:
-
“Battery metals recycling by flash Joule heating” | Science Advances | DOI: 10.1126/sciadv.adh5131
Authors: Weiyin Chen, Jinhang Chen, Ksenia Bets, Rodrigo Salvatierra, Kevin Wyss, Guanhui Gao, Chi Hun Choi, Bing Deng, Xin Wang, John Tianci Li, Carter Kittrell, Nghi La, Lucas Eddy, Phelecia Scotland, Yi Cheng, Shichen Xu, Bowen Li, Mason B. Tomson, Yimo Han, Boris Yakobson and James Tour
https://www.science.org/doi/full/10.1126/sciadv.adh5131 - Image downloads:
-
https://news-network.rice.edu/news/files/2023/09/DSCF3886.jpg
CAPTION: Jinhang Chen (left) and James Tour (Photo by Jeff Fitlow/Rice University)
https://news-network.rice.edu/news/files/2023/09/batteryrecylcing_img1-1.jpg
CAPTION: Schematic of the battery recycling process developed by Rice University scientists that uses their signature Joule-heating technique to remove the inert layer on battery metals and lower their oxidation state, making them soluble in low-concentration acid.
(Image courtesy of the Tour lab/Rice University)
- Related stories:
-
Making hydrogen from waste plastic could pay for itself:
https://news.rice.edu/news/2023/making-hydrogen-waste-plastic-could-pay-itself
Bending 2D nanomaterial could ‘switch on’ future technologies:
https://news.rice.edu/news/2023/bending-2d-nanomaterial-could-switch-future-technologies
Potential for profits gives Rice lab’s plastic waste project promise:
https://news.rice.edu/news/2023/potential-profits-gives-rice-labs-plastic-waste-project-promise
Cars could get a ‘flashy’ upgrade:
https://news.rice.edu/news/2022/cars-could-get-flashy-upgrade
Rice flashes new life into lithium-ion anodes:
https://news.rice.edu/news/2022/rice-flashes-new-life-lithium-ion-anodes
Brushing thin films onto electrodes preserves batteries:
https://news.rice.edu/news/2022/brushing-thin-films-electrodes-preserves-batteries - Links:
-
Tour lab: https://www.jmtour.com/
Department of Chemistry: https://chemistry.rice.edu/
Yakobson Research Group: https://biygroup.blogs.rice.edu/
Department of Materials Science and NanoEngineering: msne.rice.edu
George R. Brown School of Engineering: https://engineering.rice.edu
Wiess School of Natural Sciences: https://naturalsciences.rice.edu/
- About Rice:
-
Located on a 300-acre forested campus in Houston, Rice University is consistently ranked among the nation’s top 20 universities by U.S. News & World Report. Rice has highly respected schools of Architecture, Business, Continuing Studies, Engineering, Humanities, Music, Natural Sciences and Social Sciences and is home to the Baker Institute for Public Policy. With 4,552 undergraduates and 3,998 graduate students, Rice’s undergraduate student-to-faculty ratio is just under 6-to-1. Its residential college system builds close-knit communities and lifelong friendships, just one reason why Rice is ranked No. 1 for lots of race/class interaction and No. 4 for quality of life by the Princeton Review. Rice is also rated as a best value among private universities by Kiplinger’s Personal Finance.

