New technology developed by Rice University engineers could lower the cost of capturing carbon dioxide from all types of emissions, a potential game-changer for both industries looking to adapt to evolving greenhouse gas standards and for the emergent energy-transition economy.
According to a study published in Nature, the system from the lab of chemical and biomolecular engineer Haotian Wang can directly remove carbon dioxide from sources ranging from flue gas to the atmosphere by using electricity to induce a water-and-oxygen-based electrochemical reaction. This technological feat could turn direct air capture from fringe industry ⎯ there are only 18 plants currently in operation worldwide ⎯ into a promising front for climate change mitigation.
Most carbon-capture systems involve a two-step process: First, high- pH liquids are used to separate the carbon dioxide, which is acidic, from mixed-gas streams such as flue gas. Next, the carbon dioxide is regenerated from the solution through heating or by injecting a low-pH liquid.
“Once the carbon dioxide is trapped in these solvents, you have to regenerate it,” Wang said.

“Traditional amine scrubbing methods require temperatures of 100-200 degrees Celsius (212-392 Fahrenheit). For calcium carbonate-based processes you need temperatures as high as 900 Celsius (1652 Fahrenheit).
“There are literally no chemicals produced or consumed with our process. We also don’t need to heat up or pressurize our device, we just need to plug it into a power outlet and it will work.”
Another drawback of current carbon-capture technologies is their reliance on large-scale, centralized infrastructure. By contrast, the system developed in the Wang lab is a scalable, modular, point-of-use concept that can be adapted to a variety of scenarios.
“The technology can be scaled up to industrial settings ⎯ power plants, chemical plants ⎯ but the great thing about it is that it allows for small-scale use as well: I can even use it in my office,” Wang said. “We could, for example, pull carbon dioxide from the atmosphere and continuously inject that concentrated gas into a greenhouse to stimulate plant growth. We’ve heard from space technology companies interested in using the device on space stations to remove the carbon dioxide astronauts exhale.”
The reactor developed by Wang and his team can continuously remove carbon dioxide from a simulated flue gas with efficiency above 98% using a relatively low electricity input.
“The electricity used to power a 50-watt lightbulb for an hour will yield 10 to 25 liters of high-purity carbon dioxide,” said Peng Zhu, a chemical and biomolecular engineering graduate student and lead author on the study.
Wang noted that the process has “no carbon footprint or a very limited footprint” if powered by electricity from renewable sources such as solar or wind.
“This is great news considering that renewable electricity is becoming more and more cost-effective,” Wang said.
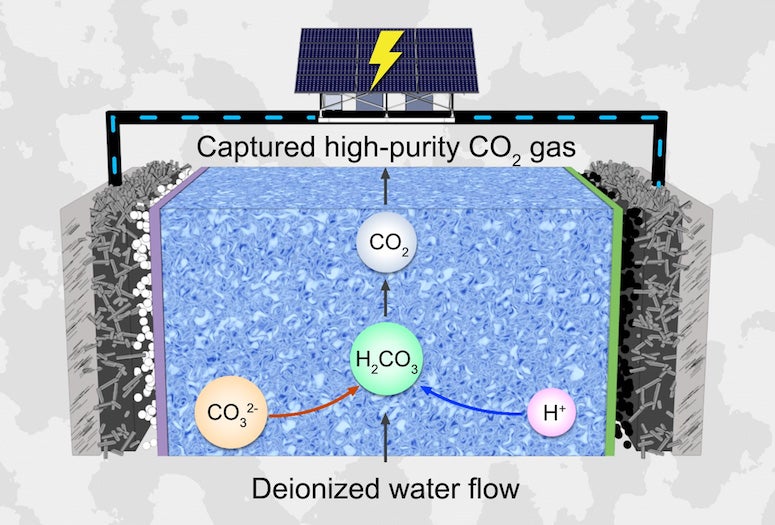
The reactor consists of a cathode set up to perform oxygen reduction, an oxygen evolution reaction-performing anode and a compact yet porous solid- electrolyte layer that allows efficient ion conduction. An earlier version of the reactor was used to reduce carbon dioxide into pure liquid fuels and reduce oxygen into pure hydrogen peroxide solutions.
“Previously, our group focused mainly on carbon dioxide utilization,” Zhu said. “We worked on producing pure liquid products like acetic acid, formic acid, etc.”
According to Wang, Zhu observed during the research process that gas bubbles flowed out of the reactor’s middle chamber along with the liquids.
“At the beginning, we didn’t pay a lot of attention to this phenomenon,” Wang said. “However, Peng observed that if we applied more current there were more bubbles. That’s a direct correlation, which means that something not random is happening.”
The researchers realized that the alkaline interface generated during reduction reactions at the reactor’s cathode side interacted with carbon dioxide molecules to form carbonate ions. The carbonate ions migrate into the reactor’s solid-electrolyte layer where they combine with protons resulting from water oxidation at the anode side, forming a continuous flow of high-purity carbon dioxide.
“We randomly discovered this phenomenon during our previous studies,” Wang said. “We then tuned and optimized the technology for this new project and new application. We’ve spent years of continuous work on this type of electrochemical device.
“Scientific discovery often requires this patient, continuous observation and the curiosity to learn what’s really going on, the choice not to neglect those phenomena that don’t necessarily fit in the experimental frame.”
Wang is the William Marsh Rice Trustee Chair and assistant professor of chemical and biomolecular engineering, materials science and nanoengineering, and chemistry, and has recently been promoted to associate professor with tenure, effective July 1.
The National Science Foundation (2029442), the Robert A. Welch Foundation (C-2051-20200401) and the David and Lucile Packard Foundation (2020-71371) supported the research.
-30-
- Peer-reviewed paper:
-
Continuous carbon capture in an electrochemical solid-electrolyte reactor | Nature | DOI: 10.1038/s41586-023-06060-1
Authors: Peng Zhu, Zhen-Yu Wu, Ahmad Elgazzar, Chagxin Dong, Tae-Ung Wi, Feng-Yang Chen, Yang Xia, Yuge Feng, Mohsen Shakouri, Jung Yoon Kim, Zhiwei Fang, T. Alan Hatton and Haotian Wang
https://doi.org/10.1038/s41586-023-06060-1 - Video URL:
-
https://youtu.be/7sGoU91RcEE
(Video by Brandon Martin/Rice University)
- Image downloads:
-
https://news-network.rice.edu/news/files/2023/05/230515_Wang_NATURE_PZHW_LG.jpg
CAPTION: Peng Zhu (left) and Haotian Wang beside their carbon-capture device prototype. (Photo by Jeff Fitlow/Rice University)https://news-network.rice.edu/news/files/2023/05/230515_Wang_NATURE_device2.jpg
CAPTION: The reactor is made up of a cathode set up to perform oxygen reduction, an oxygen evolution reaction-performing anode and a compact yet porous solid-electrolyte layer that allows efficient ion conduction. (Photo by Jeff Fitlow/Rice University) - Related stories:
-
Rice lab advances water-splitting catalysts:
https://news.rice.edu/news/2022/rice-lab-advances-water-splitting-catalysts
Rice process aims to strip ammonia from wastewater:
https://news.rice.edu/news/2022/rice-process-aims-strip-ammonia-wastewater#:~:text=1%2DWEB.jpg-,Rice%20University%20engineers%20have%20designed%20a%20catalyst%20of%20ruthenium%20atoms,traditional%20industrial%20production%20of%20ammonia.
Carbon monoxide reduced to valuable chemical:
https://news.rice.edu/news/2021/carbon-monoxide-reduced-valuable-chemical#:~:text=Rice%20University%20engineers%20are%20turning,out%20a%20highly%20purified%20product.
Funding flows into liquid fuel strategy:
https://news.rice.edu/news/2020/funding-flows-liquid-fuel-strategy
Water + air + electricity = hydrogen peroxide:
https://news2.rice.edu/2019/10/10/water-air-electricity-hydrogen-peroxide-2/
Rice reactor turns greenhouse gas into pure liquid fuel:
https://news2.rice.edu/2019/09/03/rice-reactor-turns-greenhouse-gas-into-pure-liquid-fuel-2/
- Links:
-
The Wang Group: https://wang.rice.edu
Department of Chemical and Biomolecular Engineering: https://chbe.rice.edu
George R. Brown School of Engineering: https://engineering.rice.edu
- About Rice:
-
Located on a 300-acre forested campus in Houston, Rice University is consistently ranked among the nation’s top 20 universities by U.S. News & World Report. Rice has highly respected schools of Architecture, Business, Continuing Studies, Engineering, Humanities, Music, Natural Sciences and Social Sciences and is home to the Baker Institute for Public Policy. With 4,552 undergraduates and 3,998 graduate students, Rice’s undergraduate student-to-faculty ratio is just under 6-to-1. Its residential college system builds close-knit communities and lifelong friendships, just one reason why Rice is ranked No. 1 for lots of race/class interaction and No. 4 for quality of life by the Princeton Review. Rice is also rated as a best value among private universities by Kiplinger’s Personal Finance.