Researchers at Rice University have successfully synthesized a group of natural compounds known as fusicoccanes. The molecules, found in various living organisms, exhibit diverse biological activities, including the ability to modulate protein-protein interactions within biological systems.
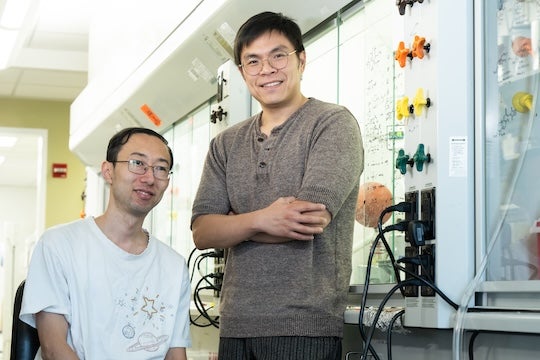
The study led by Hans Renata, associate professor of chemistry, marks a significant advancement in chemical synthesis techniques, leveraging modern organic chemistry and engineered enzymes. The research was published May 6 in the journal Nature Chemistry.
The team synthesized 10 distinct fusicoccanes by a novel strategy that combines the synthesis of the compounds’ core structures through organic chemistry with the precise decoration of functional groups using enzymes.
“Our approach represents a fusion of traditional synthetic methods with cutting-edge enzymatic catalysis,” Renata said. “By harnessing the power of engineered enzymes, we’ve achieved the synthesis of these complex molecules and also paved the way for further chemical modifications.”
The researchers encountered challenges during the enzymatic phase of the synthesis as the chosen enzymes were initially unstable and led to undesirable side products. But through rigorous experimentation and enzyme engineering, they identified improved enzyme variants tailored to the synthetic process.
“This work demonstrates the importance of enzyme engineering in enabling the synthesis of biologically relevant compounds,” Renata said. “Our findings highlight the potential of a hybrid synthetic strategy for the development of new molecules with diverse applications.”
In addition to their direct application as modulators of protein-protein interactions, the synthesized compounds open avenues for exploring new drug candidates and understanding biological processes, lead author and postdoctoral researcher Yanlong Jiang said.
“Our methodology could inspire similar innovations in the synthesis of other valuable molecules, driving advancements in various fields,” Jiang said.
The research was supported by the Alfred P. Sloan Foundation, U.S. Department of Health and Human Services, National Institute of General Medical Sciences, and Cancer Prevention and Research Institute of Texas (CPRIT) under NCHEM-23081635-T. Renata in 2022 joined Rice as a CPRIT scholar.