In the global race to decarbonize, hydrogen stands out as one of the most promising clean fuels. But despite its potential to power industries and transportation without emitting carbon, producing hydrogen sustainably in a water electrolyzer has been limited by the high cost and scarcity of one critical ingredient: iridium.
Now, a team of researchers at Rice University has developed a new catalyst that dramatically reduces the amount of iridium needed in proton exchange membrane (PEM) water electrolyzers, a key technology for generating green hydrogen from water. Their innovation — an iridium-stabilized ruthenium oxide catalyst that uses just one-sixth as much iridium as conventional systems — maintains industrial-level performance for more than 1,500 hours of continuous operation. The research was recently published in Nature Nanotechnology.

“This is a significant step toward making green hydrogen more accessible and scalable,” said Haotian Wang, associate professor of chemical and biomolecular engineering at Rice. “By reducing iridium use by over 80%, we’re addressing one of the biggest economic and supply chain bottlenecks in the hydrogen economy.”
Current PEM electrolyzers rely heavily on iridium because it is one of the few metals that can withstand the harsh, acidic conditions needed to split water efficiently. But iridium is among the rarest elements on Earth — its price is currently around $160 per gram — and global production is extremely limited.
“Without reducing iridium consumption, the projected demand from electrolyzers alone could exceed 75% of the world’s annual supply,” Wang said. “That’s simply not sustainable if we’re serious about scaling hydrogen production.”
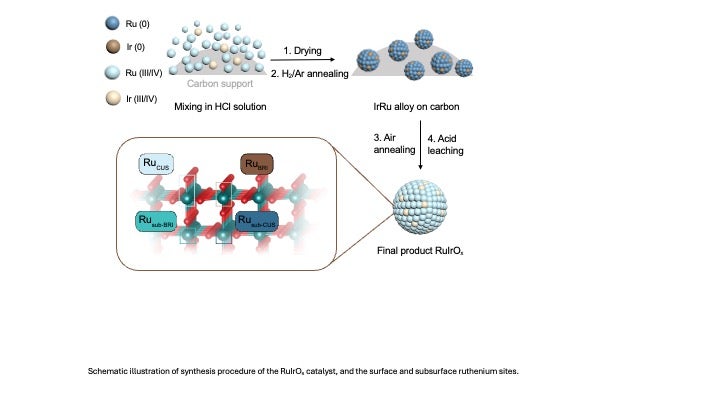
To tackle this challenge, the Rice team, working with industrial collaborators at De Nora Tech, combined density functional theory and Monte Carlo simulations to design a new atomic structure where iridium atoms are strategically embedded within a ruthenium oxide (RuO2) lattice. This arrangement provides stability from beneath the surface, an unexpected discovery that allowed the researchers to achieve durable performance with far less iridium.
“Our simulations revealed that iridium atoms in the subsurface layer play a critical role,” said Thomas Senftle, the William Marsh Rice Trustee Associate Professor of Chemical and Biomolecular Engineering at Rice. “They help protect the ruthenium atoms above them from dissolving under extreme electrochemical conditions, essentially reinforcing the lattice from within.”
Experimentally, the team synthesized a catalyst dubbed Ru6IrOₓ, representing a ruthenium-to-iridium atomic ratio of 6-to-1. The material demonstrated exceptional long-term stability, sustaining 2 amperes per square centimeter of current density (an industrial benchmark) for over 1,500 hours with minimal degradation.
“The key is achieving a uniform distribution of iridium throughout the ruthenium oxide structure,” Senftle said. “That uniformity promotes stability because iridium helps to stabilize neighboring ruthenium atoms in the oxide lattice.”
The catalyst’s performance was also verified under industrial testing standards in a 25-square-centimeter PEM electrolyzer operated by De Nora Tech. Under real-world conditions, the Rice-designed catalyst maintained stable operation at high current and temperature, matching the activity of pure iridium catalysts despite using a fraction of the metal.
“Our results show that we don’t need iridium-rich catalysts to achieve durability,” Wang said. “This opens the door to mass production of cost-effective, high-performance PEM electrolyzers.”
The economic implications are striking. An economic analysis by the team showed that replacing conventional iridium oxide with the Ru6IrOₓ catalyst could cut the anode catalyst cost by more than 80%, while also reducing sensitivity to iridium price fluctuations.
Beyond economics, the research offers a new paradigm for catalyst design: stabilizing materials from within rather than shielding them from the surface.
“This work highlights how theory and experiment can work hand in hand,” Senftle said. “By combining atomic-scale simulations with rigorous experimental testing, we’ve been able to pinpoint how a small amount of iridium can stabilize the entire oxide lattice.”
The breakthrough could help accelerate global deployment of PEM electrolyzers, which are favored for their efficiency and compact design but hampered by cost. As nations and companies invest billions into hydrogen hubs and decarbonization projects, innovations like Rice’s low-iridium catalyst are poised to play a critical role.
“This is about removing the barriers to entry for the hydrogen economy,” Wang said. “If we can make electrolyzers cheaper, more durable and less dependent on scarce materials, hydrogen can become a truly global, renewable fuel.”
The research was supported by the Robert A. Welch Foundation, the David and Lucile Packard Foundation and the National Science Foundation. The 25-square-centimenter reactor testing was performed in collaboration with De Nora Tech (a subsidiary of Industrie De Nora S.p.A.) and advanced microscopy and spectroscopy were conducted at Oak Ridge National Laboratory and Brookhaven National Laboratory.