by Patrick Kurp
Special to Rice News

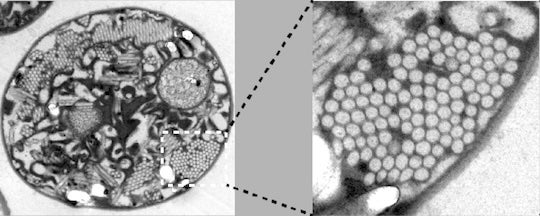
Gas vesicles are hollow structures made of protein found in the cells of certain microorganisms, and researchers at Rice University believe they can be programmed for use in biomedical applications.
“Inside cells, gas vesicles are packed in a beautiful honeycomb pattern. How this pattern is formed has never been thoroughly understood. We are presenting the first identification of a protein that can regulate this patterning, and we believe this will be a milestone in molecular microbiology,” said George Lu, assistant professor of bioengineering and a Cancer Prevention and Research Institute of Texas scholar.
Lu and colleagues have published their findings in a paper published in Nature Microbiology. The lead author is Zongru Li, a fourth-year bioengineering doctoral student in Lu’s Laboratory for Synthetic Macromolecular Assemblies.
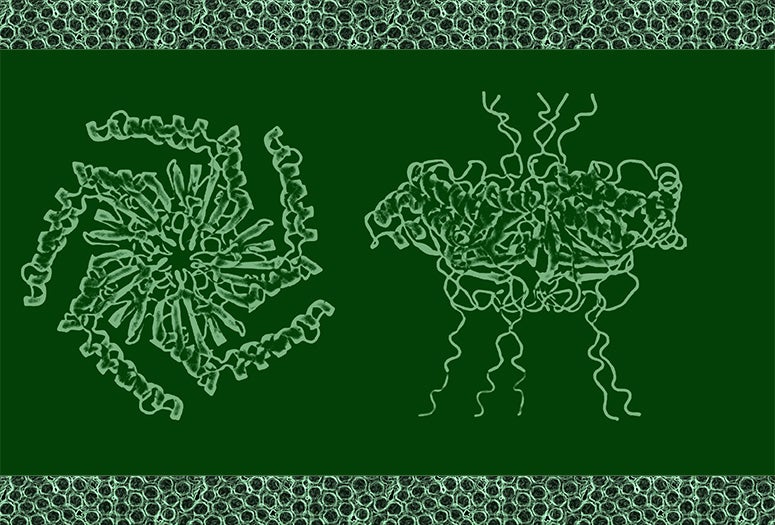
“Gas vesicles are cylindrical tubes closed by conical end caps,” Li said. “They provide buoyancy within the cells of their native hosts.”
The vesicles are found naturally in five phyla of bacteria and two groups of the archaea (single-cell organisms). Most are restricted to planktonic microorganisms often found in fresh-water ponds. The recent engineering of vesicles has led to
several applications, including reporter gene imaging, acoustic control and payload delivery.
Co-author Yifan Dai, assistant professor of biomedical engineering at McKelvey School of Engineering at Washington University in Saint Louis, said they were drawn to the research with the question of why the vesicles can form in the honeycomb pattern.
With help from his WashU colleague Alex Holehouse and colleagues from Duke University, Ashutosh Chilkoti and Lingchong You, the team of researchers found that this pattern is the most efficient use of space and the cluster form plays a part in how it functions. Most notably, these protein clusters formed in subsaturated solution, a previously identified new form of biological structure, and that drives the organization of these vesicles. Bottom line, they found the function behind this mysterious new form.
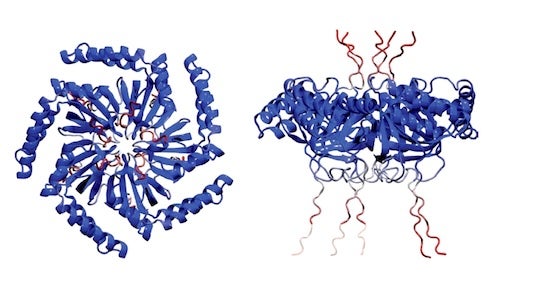
“These teams led by Lu lab found that a unique form of protein clusters exclusively assembled in subsaturated solution drives the clustering behaviors,” said Dai. This adds to the line of evidence on how phase transition affects cellular organization and cellular functions, he added.
Lu and his team, using genetic, biochemical and imaging approaches, are exploring the protein nanostructures. Gas vesicles stabilize the air bubbles inside the bacterial cytosol ⎯ the fluids inside the cells ⎯ and provide a liquid-gas interface which can be used for ultrasound or MRI contrast.
“In our lab, we are leveraging the power of synthetic biology to expand the applications of these protein nanostructures,” Li said. “By engineering genes and cells, we aim to build gas vesicles that perform even more efficiently in biotechnological and biomedical applications.”

Li earned his B.S. in chemical engineering from the University of Rochester in 2018 and his M.S. in biotechnology from Northwestern University in 2020.
Co-authors of the paper are Andrew Anderson, Manuel Iburg, Qionghua Shen, postdoctoral researchers in BIOE at Rice; Richard Lin ’23 BIOE, sustainable solutions and innovation analyst, NRG; Brandon Zimmer ’23 BIOE; Matthew Meyer, Rice electron microscopy research scientist; Yifan Dai and Alex Holehouse, assistant professors of biomedical engineering at WashU; Lingchong You, James L. Meriam Distinguished Professor of Biomedical Engineering at Duke; Ashutosh Chilkoti, the Alan L. Kaganov Distinguished Professor of Biomedical Engineering at Duke; and Emery Usher, postdoctoral researcher in biochemistry and molecular biology at WashU.
The research was supported by the Cancer Prevention and Research Institute of Texas, the National Institutes of Health (R00 EB024600, R21 EB033607), the Welch Foundation, G. Harold and Leila Y. Mathers Foundation, Hearing Health Foundation, John S. Dunn Foundation, German Research Foundation, W.M. Keck Foundation, the Institute of Biosciences and Bioengineering at Rice and the Air Force Office of Scientific Research (FA9550-20-1-0241).
- Peer-reviewed paper:
-
“Phase transition of GvpU regulates gas vesicle clustering in bacteria” | Nature Microbiology | DOI: 10.1038/s41564-024-01648-3
Authors: Zongru Li, Qionghua Shen, Emery Usher, Andrew Anderson, Manuel Iburg, Richard Lin, Brandon Zimmer, Matthew Meyer, Alex Holehouse, Lingchong You, Ashutosh Chilkoti, Yifan Dai and George Lu
https://www.nature.com/articles/s41564-024-01648-3 - Image downloads:
-
https://news-network.rice.edu/news/files/2024/03/Lu-et-al-img1-ed8bc22cc94ecbf2.jpg
CAPTION: Gas vesicles inside a microorganism. (Image courtesy of the Lu lab/Rice University)
https://news-network.rice.edu/news/files/2024/03/img-7f957c33dee15e01.jpg
CAPTION: Simulated configuration of the protein structure. (Image courtesy of the Lu lab/Rice University)
https://news-network.rice.edu/news/files/2024/03/00_Lu_img1-4d7124104628632a.jpg
CAPTION: Zongru Li (left) and George Lu (Photo by Anna Stafford/Rice University)
https://news-network.rice.edu/news/files/2024/03/IMG_1691-b3b08dc64380daea.jpeg
CAPTION: Zongru Li (left) and George Lu (Photo by Anna Stafford/Rice University)