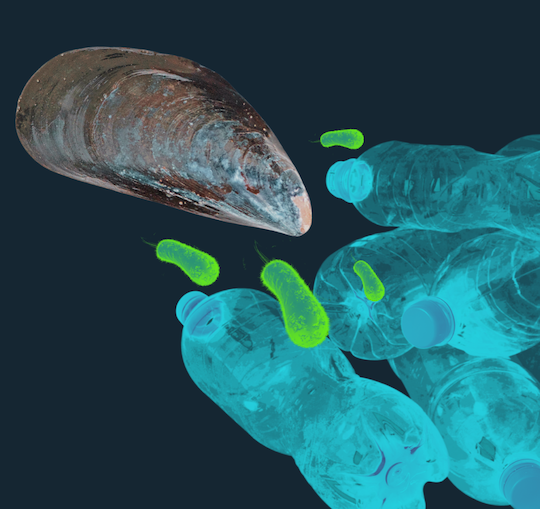
Rice University scientists have tapped into nature’s adhesive genius — the sticky power of mussels — to create bioengineered microorganisms with powerful cling that could help transform environmental cleanup. By combining this amplified sticking force with an enzyme that breaks down harmful plastics, their discovery offers a potential new tool for tackling plastic pollution. The research, published in Small Methods, could also curb biofouling, addressing long-standing challenges in industries ranging from shipping to medicine.
The U.S. produces about 40 million tons of plastic waste annually, according to the Environmental Protection Agency, with polyethylene terephthalate (PET) accounting for 64%. PET, a plastic often found in packaging, is notoriously resistant to degradation, taking centuries to decompose. The team’s innovation allowed it to create adhesive bacteria and proteins that could help countries worldwide more efficiently decompose PET.
“Very excitingly, our research holds promise for addressing the growing problem of plastic pollution in the U.S. and across the globe,” said study leader Han Xiao, the director of Rice’s Synthesis X Center, an associate professor of chemistry, biosciences and bioengineering and a Cancer Prevention and Research Institute of Texas (CPRIT) scholar.
Tackling plastic pollution

The engineered bacteria were designed using genetic code expansion technology, incorporating a natural amino acid called 3,4-dihydroxyphenylalanine (DOPA), responsible for mussels’ adhesive properties. The researchers significantly enhanced their ability to bind to PET surfaces by modifying the bacteria with DOPA.
The altered bacteria, tested on PET samples at 37 degrees Celsius, demonstrated a 400-fold increase in adhesion. This cohesive bacteria was united with an enzyme called polyethylene terephthalate hydrolase to break the material into smaller, more manageable fragments, resulting in what the researchers call a significant amount of degradation of the plastics overnight.
This innovative approach could provide a novel solution to plastic recycling, offering a faster and more efficient way to reduce plastic waste and its environmental impact.
“Our approach underscores the innovative utility of genetic code expansion in material and cellular engineering. It can potentially transform bioengineering applications and solve real-world problems,” Xiao said.
Combating biofouling

In addition to addressing plastic pollution, the research offers potential solutions to biofouling, the unwanted accumulation of microorganisms, plants, algae, and small animals on submerged surfaces that can damage ships’ hulls, underwater structures, and pipes.
The DOPA-modified proteins showed strong bonding capabilities to organic and metallic surfaces, creating a barrier that prevents the accumulation of microorganisms and other materials.
Moreover, the researcher’s discovery has broad applications, including the health care field. For example, it can be used to prevent bacterial growth on medical devices, making them safer and more effective, the researchers said.
“This will open up new avenues for leveraging these interactions to develop smart material-protein conjugates for various biomedical applications like implantable medical devices, tissue engineering and drug delivery,” said Mengxi Zhang, first author of the study and a graduate student in chemistry.
Other authors include Yuda Chen, Anna Chung, Shudan Yang, Chi Hun Choi, and Sophie Zhang from Rice’s Department of Chemistry and Yimo Han from its Materials Science and Nanoengineering Department.
Grants from CPRIT, the National Institutes of Health, the Robert A. Welch Foundation, the U.S. Department of Defense, the John S. Dunn Foundation Collaborative Research Award, and the Hamill Innovation Award supported this research.