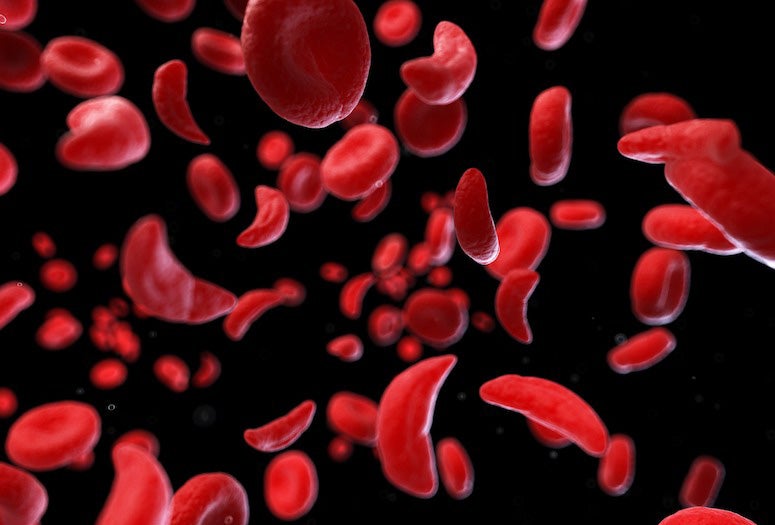
The U.S. Food and Drug Administration is about to make history today with its decision on whether or not to approve a CRISPR-based treatment for sickle cell disease, an inherited disorder that distorts the shape of red blood cells and disrupts the function of hemoglobin, the protein that carries and distributes oxygen throughout the body.
Rice University’s Gang Bao, a pioneer in nanomedicine, molecular imaging and genome editing with long-standing experience researching CRISPR-based gene correction techniques for treating sickle cell diseases and other disorders, is available to comment on the FDA decision.
“FDA approval of a gene editing-based cure for sickle cell disease is great news for patients and for the gene editing field,” Bao said. “However, there are still safety concerns, and the estimated cost is very high.”
The condition affects over 100,000 people in the U.S. and 20 million people worldwide, according to the National Heart, Lung and Blood Institute.
Bao is Rice’s Foyt Family Professor of Bioengineering and department chair, professor of chemistry, mechanical engineering and materials science and nanoengineering and a Cancer Prevention and Research Institute of Texas scholar.
To schedule an interview, contact Silvia Cernea Clark, media relations specialist, at 713-348-6728.