A new study by Rice University bioscientists explains how basic cellular structures common to most life forms collaborate to fuel growth in a model plant organism. The findings could shed light on corresponding mechanisms in human cells.

Before it is mature enough to perform photosynthesis, a developing Arabidopsis thaliana seedling relies on the reserve of fats stored inside its cells in protein-coated pouches known as lipid droplets. The energy-rich contents of these droplets are mobilized in intracellular containers called peroxisomes. The new study found that this collaboration requires an enzyme on the peroxisome that helps break down the protein coating on the lipid droplets. The enzyme ⎯ MIEL1 ⎯ was previously known to reside in the cell nucleus, where it helps regulate gene expression.
The discovery of a new role and location for MIEL1 prompted the question of whether the findings would also apply to its human counterpart, PIRH2. Additional experiments confirmed that PIRH2 associates with peroxisomes when expressed in Arabidopsis cells, according to the study published in the Proceedings of the National Academy of Sciences. Because PIRH2 plays a significant role in tumor development, a more thorough understanding of its cellular function could offer insights in cancer prevention and treatment.
In human cells, PIRH2 helps degrade p53, a well-known protein that controls the proliferation of cells with a damaged genome. The gene that encodes p53 has been dubbed “the guardian of the genome” and studied extensively for its tumor-suppressing role. Mutations that disrupt its function are found in most cancers.
“PIRH2 is one of the most highly studied of p53 regulators, so it’s directly tied to cancer, because p53 is often mutated and implicated in a variety of cancers in a lot of different organs and cell types,” said Melissa Traver, a postdoctoral research associate in the Bartel lab who is the lead author on the study.
“Because of that, it’s an interesting gene to study,” added Traver, who is also a Rice alum of the biochemistry and cell biology doctoral program. “Learning more about it could inform what we know about how cancer works and how to stop it. I never thought that I would end up reading papers about cancer in a plant lab. I started out this project trying to answer a very, very specific question about plants, and it has been extremely encouraging and rewarding to find something that’s potentially more widely applicable across systems.”

(Photo by Jeff Fitlow/Rice University)
The findings reinforced Traver’s conviction that fundamental research can yield impacts as important as application-based science.
“As a graduate student, I've spent a lot of time defending basic science and stressing its necessity and intrinsic value,” Traver said.
Together with Bonnie Bartel, Rice’s Ralph and Dorothy Looney Professor of BioSciences, Traver examined the cellular processes that unfold during Arabidopsis germination.
“For years, we've been studying peroxisomes in Arabidopsis ⎯ how they're made and what they do and why they're important,” said Bartel. “Peroxisomes play an especially critical role during germination, when the plant undergoes significant growth but is not yet mature enough to carry out photosynthesis. This means it has to use the lipids stored in the seed by the parent plant.
“We started getting interested in lipid droplets because of the close association of these two organelles ⎯ one where fats are stored and one where they’re processed. Lipid droplets have a protein coating that keeps them from coalescing with one another. We were interested in how the cells get rid of this protein, which is called oleosin.”
To find out how oleosin gets broken down, Traver engineered a version of the protein tagged with a fluorescent marker.
“We are geneticists, so when we want to understand something, we like to break it,” Bartel said. “Melissa decided to look for plants that couldn’t degrade this oleosin in the way that wild type does. Because of the fluorescent tag on the oleosin, she could see that the wildtype seedling was lit up at first. However, the fluorescence fades as the oleosin gets broken down and the lipids are consumed.”

In contrast, the mutant seedlings that cannot break down oleosin continue to display fluorescence. By sequencing their genome and comparing it to that of the wild type plant, Traver was able to identify the gene responsible for the plants’ ability to break down oleosin.
“The gene that’s no longer working in the mutant seedlings codes for MIEL1, a nuclear enzyme that helps degrade transcription factors ⎯ proteins that modulate gene expression,” Bartel said.
Traver performed more experiments to figure out whether MIEL1 accompanies lipid droplets or peroxisomes.
“The unexpected thing Melissa found is that even though it acts on lipid droplets, MIEL1 is actually localized at the peroxisome,” Bartel said. “Lipid droplets and peroxisomes are scattered throughout the cell, and you don't want to degrade the oleosin unless a lipid droplet is right next to a peroxisome. Our hypothesis is that having this enzyme on the peroxisomes is a way to ensure the right biochemistry happens right where it’s needed.”
The study’s findings suggest the enzyme-mediated interaction between peroxisomes and lipid droplets could look similar across all eukaryotic life forms.
“The next thing to do would be to carry out experiments in human cells or other animal models to see if similar mechanisms are at play,” Traver said.
The National Institutes of Health (R35GM130338, P30CA91842, UL1TR002345, R01GM129325) and the Robert A. Welch Foundation (C-1309) supported the research.
- Peer-reviewed paper:
-
The ubiquitin-protein ligase MIEL1 localizes to peroxisomes to promote seedling oleosin degradation and lipid droplet mobilization | Proceedings of the National Academy of Sciences | DOI: 10.1073/pnas.2304870120
Authors: Melissa Traver and Bonnie Bartel
https://doi.org/10.1073/pnas.2304870120 - Image downloads:
-
https://news-network.rice.edu/news/files/2023/07/230630_Bartel2_Fitlow_073.jpg
CAPTION: Bonnie Bartel (left) is Rice’s Ralph and Dorothy Looney Professor of BioSciences. Melissa Traver is a postdoctoral research associate in the Bartel lab at Rice University.
(Photo by Jeff Fitlow/Rice University)
https://news-network.rice.edu/news/files/2023/07/230630_Bartel3_Fitlow_091.jpg
CAPTION: Traver (left) and Bartel discovered that peroxisomes, the specialized intracellular structures where plant cells process lipids, are accompanied by an enzyme that breaks down the protein coating of lipid droplets, lipid-storing organelles. (Photo by Jeff Fitlow/Rice University)
https://news-network.rice.edu/news/files/2023/07/230630_Bartel4_Fitlow_079.jpg
CAPTION: Melissa Traver examines Arabidopsis thaliana seed packets.
(Photo by Jeff Fitlow/Rice University)
https://news-network.rice.edu/news/files/2023/07/BB_img1.jpg
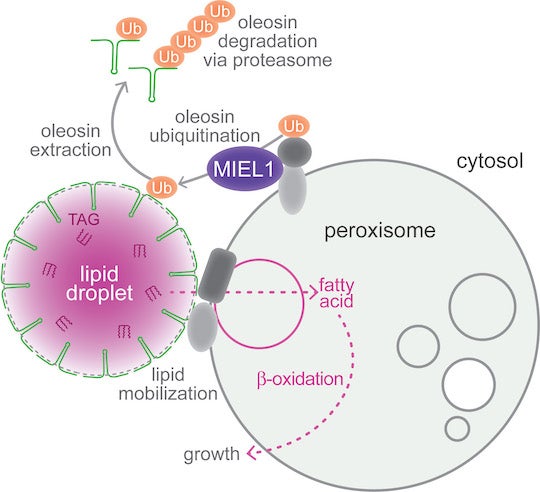
CAPTION: Working model for MIEL1 function at peroxisomes. MIEL1 localizes at peroxisomes and is necessary for removing oleosin ⎯ the protein that coats lipid droplets ⎯ during Arabidopsis thaliana seedling germination. MIEL1 signals the breakdown of oleosin (green), allowing fats stored in lipid droplets to be transferred to peroxisomes, where they are metabolized to fuel seedling growth. (Credit: Melissa Traver and Bonnie Bartel/Rice University)
https://news-network.rice.edu/news/files/2023/07/BB_img2.jpg
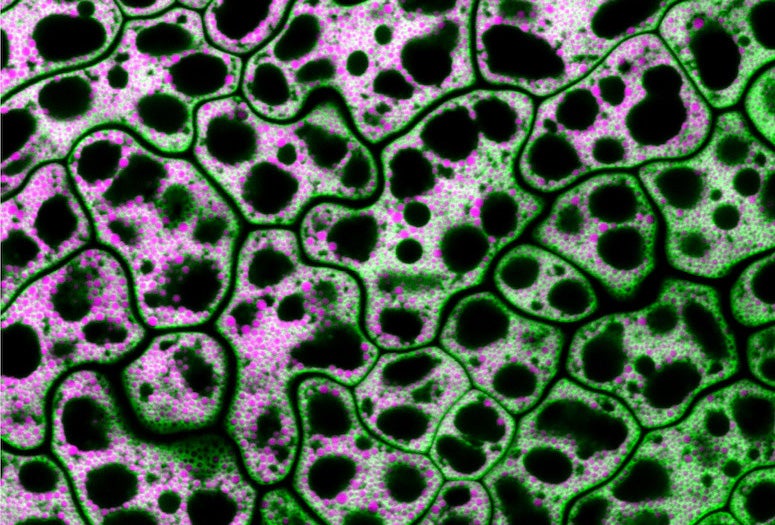
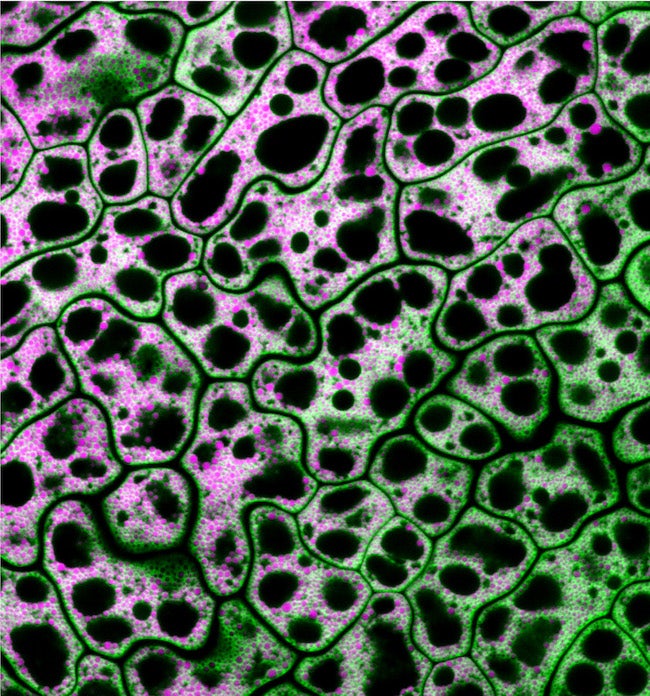
CAPTION: Pictured are cells from a MIEL1 mutant Arabidopsis seed with neutral lipids shown in magenta and a lipid droplet coat protein shown in green. Traver and Bartel used genetics and microscopy to explore the dynamic collaboration between lipid droplets and peroxisomes, organelles that store and catabolize fats, respectively. (Credit: Melissa Traver/Rice University)
https://news-network.rice.edu/news/files/2023/07/BB_img3.jpg
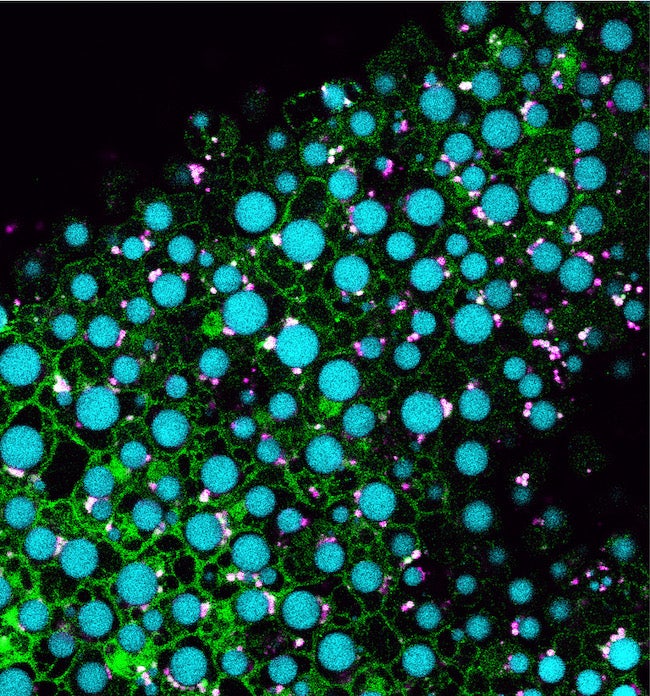
CAPTION: Pictured are cells from an Arabidopsis seedling overexpressing MIEL1 (green) with lipid droplets shown in cyan and peroxisomes shown in magenta. Traver and Bartel used genetics and microscopy to explore the dynamic collaboration between lipid droplets and peroxisomes, organelles that store and catabolize fats, respectively. Their evidence suggests that MIEL1 promotes degradation of coat proteins on lipid droplets adjacent to peroxisomes. (Credit: Melissa Traver/Rice University)
- Related stories:
-
Hidden structure found in essential metabolic machinery:
https://news.rice.edu/news/2020/hidden-structure-found-essential-metabolic-machinery - Links:
-
Bartel lab: http://www.bioc.rice.edu/~bartel/people.html
Department of BioSciences: https://biosciences.rice.edu/Wiess School of Natural Sciences: https://naturalsciences.rice.edu
- About Rice:
-
Located on a 300-acre forested campus in Houston, Rice University is consistently ranked among the nation’s top 20 universities by U.S. News & World Report. Rice has highly respected schools of Architecture, Business, Continuing Studies, Engineering, Humanities, Music, Natural Sciences and Social Sciences and is home to the Baker Institute for Public Policy. With 4,552 undergraduates and 3,998 graduate students, Rice’s undergraduate student-to-faculty ratio is just under 6-to-1. Its residential college system builds close-knit communities and lifelong friendships, just one reason why Rice is ranked No. 1 for lots of race/class interaction and No. 4 for quality of life by the Princeton Review. Rice is also rated as a best value among private universities by Kiplinger’s Personal Finance.