HOUSTON – (April 5, 2022) – Here’s another thing to do with that mountain of used plastic: make it soak up excess carbon dioxide.

What seems like a win-win for a pair of pressing environmental problems describes a Rice University lab’s newly discovered chemical technique to turn waste plastic into an effective carbon dioxide (CO2) sorbent for industry.
Rice chemist James Tour and co-lead authors Rice alumnus Wala Algozeeb, graduate student Paul Savas and postdoctoral researcher Zhe Yuan reported in the American Chemical Society journal ACS Nano that heating plastic waste in the presence of potassium acetate produced particles with nanometer-scale pores that trap carbon dioxide molecules.
These particles can be used to remove CO2 from flue gas streams, they reported.
“Point sources of CO2 emissions like power plant exhaust stacks can be fitted with this waste-plastic-derived material to remove enormous amounts of CO2 that would normally fill the atmosphere,” Tour said. “It is a great way to have one problem, plastic waste, address another problem, CO2 emissions.”

A current process to pyrolyze plastic known as chemical recycling produces oils, gases and waxes, but the carbon byproduct is nearly useless, he said. However, pyrolyzing plastic in the presence of potassium acetate produces porous particles able to hold up to 18% of their own weight in CO2 at room temperature.
In addition, while typical chemical recycling doesn’t work for polymer wastes with low fixed carbon content in order to generate CO2 sorbent, including polypropylene and high- and low-density polyethylene, the main constituents in municipal waste, those plastics work especially well for capturing CO2 when treated with potassium acetate.
The lab estimates the cost of carbon dioxide capture from a point source like post-combustion flue gas would be $21 a ton, far less expensive than the energy-intensive, amine-based process in common use to pull carbon dioxide from natural gas feeds, which costs $80-$160 a ton.

Like amine-based materials, the sorbent can be reused. Heating it to about 75 degrees Celsius (167 degrees Fahrenheit) releases trapped carbon dioxide from the pores, regenerating about 90% of the material’s binding sites.
Because it cycles at 75 degrees Celsius, polyvinyl chloride vessels are sufficient to replace the expensive metal vessels that are normally required. The researchers noted the sorbent is expected to have a longer lifetime than liquid amines, cutting downtime due to corrosion and sludge formation.
To make the material, waste plastic is turned into powder, mixed with potassium acetate and heated at 600 C (1,112 F) for 45 minutes to optimize the pores, most of which are about 0.7 nanometers wide. Higher temperatures led to wider pores. The process also produces a wax byproduct that can be recycled into detergents or lubricants, the researchers said.
Co-authors of the paper are Rice alumnus Zhe Wang and research scientist Carter Kittrell, and graduate student Jacklyn Hall and Praveen Bollini, an assistant professor of chemical and biomolecular engineering, both of the University of Houston. Tour is the T.T. and W.F. Chao Chair in Chemistry as well as a professor of materials science and nanoengineering.
The Department of Energy (DE-F0031794) and Saudi Aramco supported the research.
- Peer-reviewed paper
-
Plastic Waste Product Captures Carbon Dioxide in Nanometer Pores: https://pubs.acs.org/doi/10.1021/acsnano.2c00955
- Images for download
-

https://news-network.rice.edu/news/files/2022/03/0714_CAPTURE-1-web.jpg
A plastic jug is fodder for a material developed at Rice University that turns waste plastic into a material that absorbs carbon dioxide. The lab is targeting flue gases that now require a far more complex process to sequester carbon dioxide. (Credit: Jeff Fitlow/Rice University)

https://news-network.rice.edu/news/files/2022/03/0714_CAPTURE-3-web.jpg
Rice University graduate student Paul Savas feeds raw plastic into a crusher to prepare it for pyrolysis, or heating in an inert atmosphere. Pyrolyzing the material in the presence of potassium salts turns it into a material that sequesters carbon dioxide from flue gas. (Credit: Jeff Fitlow/Rice University)

https://news-network.rice.edu/news/files/2022/03/0714_CAPTURE-2-WEB.jpg
A Rice University chemist prepares to heat plastic powder combined with potassium acetate to turn it into porous particles that absorb carbon dioxide. (Credit: Jeff Fitlow/Rice University)

https://news-network.rice.edu/news/files/2022/03/0714_CAPTURE-4a-WEB.jpg
Rice University chemists modify waste plastic to absorb carbon dioxide from flue gas streams more efficiently than current processes. From left: Paul Savas, James Tour and Zhe Yuan. (Credit: Jeff Fitlow/Rice University)

https://news-network.rice.edu/news/files/2022/03/0714_CAPTURE-5-WEB.jpg
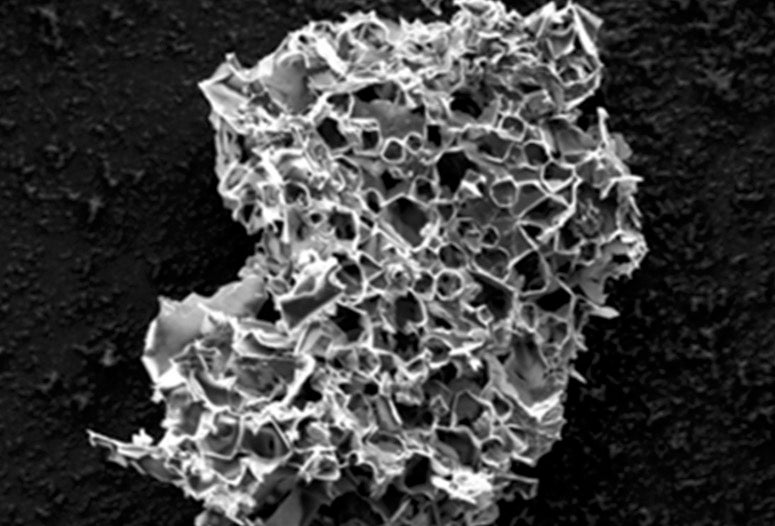
Pores in this micron-scale particle, the result of pyrolyzing in the presence of potassium acetate, are able to sequester carbon dioxide from streams of flue gas. Rice University scientists say the process could be a win-win for a pair of pressing environmental problems. (Credit: Tour Group/Rice University)
- Related links
-
Tour Group: https://www.jmtour.com
Department of Chemistry: https://chemistry.rice.edu
Wiess School of Natural Sciences: https://naturalsciences.rice.edu
Follow Rice News and Media Relations via Twitter @RiceUNews.
- About Rice
-
Located on a 300-acre forested campus in Houston, Rice University is consistently ranked among the nation’s top 20 universities by U.S. News & World Report. Rice has highly respected schools of Architecture, Business, Continuing Studies, Engineering, Humanities, Music, Natural Sciences and Social Sciences and is home to the Baker Institute for Public Policy. With 4,052 undergraduates and 3,484 graduate students, Rice’s undergraduate student-to-faculty ratio is just under 6-to-1. Its residential college system builds close-knit communities and lifelong friendships, just one reason why Rice is ranked No. 1 for lots of race/class interaction and No. 1 for quality of life by the Princeton Review. Rice is also rated as a best value among private universities by Kiplinger’s Personal Finance.