You can keep your best guesses. Engineers at Rice University’s George R. Brown School of Engineering are starting to understand exactly what goes on when doctors pump contrast agents into your body for an MRI scan.
In a new study that could lead to better scans, a Rice-led team digs deeper via molecular simulations that, unlike earlier models, make absolutely no assumptions about the basic mechanisms at play when gadolinium agents are used to highlight soft tissues.
The study led by Rice chemical and biomolecular engineer Philip Singer, former associate research professor Dilip Asthagiri, now of Oak Ridge National Laboratory, and graduate student Thiago Pinheiro dos Santos appears in Physical Chemistry Chemical Physics.
It employs the sophisticated models first developed at Rice for oil and gas studies to conclusively analyze how hydrogen nuclei at body temperatures “relax” under nuclear magnetic resonance (NMR), the technology used by magnetic resonance imaging, aka MRI.

Doctors use MRI to “see” the state of soft tissues, including the brain, in a patient by inducing magnetic moments in the hydrogen nuclei of water molecules to align with the magnetic field, a process that can be manipulated when gadolinium agents are in the vicinity. The device detects bright spots when the aligned nuclei relax back to thermal equilibrium following an excitation. The faster they relax, the brighter the contrast.
Gadolinium molecules are naturally paramagnetic and sensitive to magnetic excitation. Because they’re toxic, they are usually chelated when part of a contrast agent. “A chelate basically hugs the gadolinium and protects your body from directly interacting with the metal,” Pinheiro dos Santos said. “We’re asking, exactly how do these molecules behave?”
Though gadolinium-based contrast agents are injected by the ton into patients each year, how they work on a molecular level has never been fully understood.
“Going back 40 years, in the NMR field people assumed liquid water is just a collection of marbles moving about, and the dipoles in the marbles randomly reorient,” Asthagiri said.
But such assumptions are limiting, he said. “What Thiago does with his explicit simulation is show how the water network evolves in time,” Asthagiri said. “These are complicated, computationally intensive calculations.”
The Rice simulations make use of highly refined, polarizable force fields to study the phenomenon in detail, and that required intensive GPU-accelerated computing.
The team validated its molecular dynamics approach with experimental data by co-author Steven Greenbaum, a professor of physics at Hunter College in the City University of New York, whose lab specializes in NMR measurements of ionic and molecular transport processes in condensed matter.
The simulations revealed distinct differences in how the inner and outer shells of water molecules around gadolinium respond to thermal excitation. “The inner shell is the group of eight or nine water molecules around gadolinium,” Pinheiro dos Santos said. “They’re strongly attached to the gadolinium and they stay there for a long time, a few nanoseconds. The outer shell encompasses all of the remaining water molecules.”

The researchers found that while the structure of the inner shell does not change between 41 and 98.6 degrees Fahrenheit, its dynamics are very susceptible to thermal effects. They also discovered that temperature greatly affects the self-diffusivity of molecules in the gadolinium-water simulations in a way that affects outer-shell relaxation.
“Overall, these discoveries open a new way to elucidate how contrast agents respond at human body conditions during an MRI scan,” Singer said. “By better understanding this, one can develop new, safer and more sensitive contrast agents, as well as use simulations to enhance the interpretation of MRI data.”
He said future studies will examine chelated gadolinium complexes in fluids that are more representative of cellular interiors.
Co-authors of the paper are Rice alumnus Arjun Valiya Parambathu, now a postdoctoral researcher at the University of Delaware; Carla Fraenza and Casey Walsh of Hunter College; and Walter Chapman, the William W. Akers Professor of Chemical and Biomolecular Engineering at Rice.
The Robert A. Welch Foundation (C-1241), the Ken Kennedy Institute, the Rice University Creative Ventures Fund and the Rice University Consortium on Processes in Porous Media supported the research. Research at Oak Ridge National Laboratory is supported under contract DE-AC05-00OR22725 from the U.S. Department of Energy to UT-Battelle LLC.
- Peer-reviewed research
-
Thermal and concentration effects on 1H NMR relaxation of Gd3+-aqua using MD simulations and measurements: https://pubs.rsc.org/en/content/articlelanding/2022/cp/d2cp04390d
- Video
-
Video produced by Thiago Pinheiro dos Santos
- Images for download
-
https://news-network.rice.edu/news/files/2022/11/1124_NMR-1-web.jpg
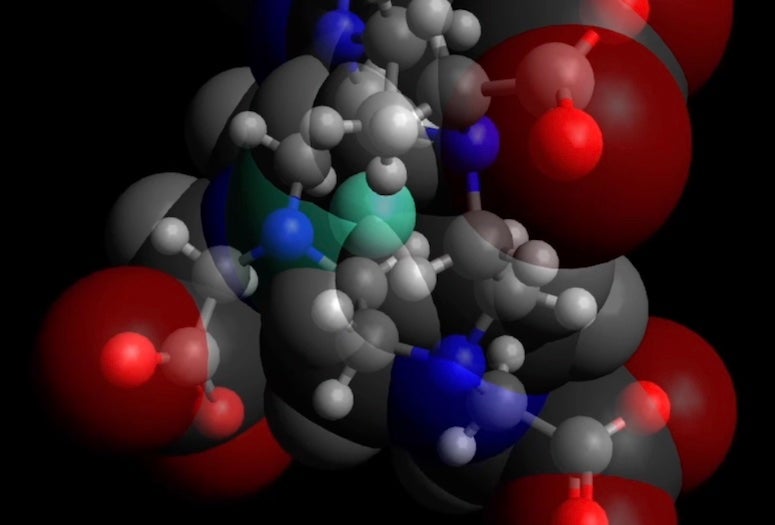
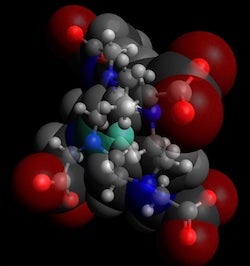
Simulations by Rice University engineers have revealed details about the molecular interactions between gadolinium contrast agents used in MRI scans and their liquid environment. In this model, green gadolinium is surrounded by blue chelate ions, themselves surrounded by water (gray oxygen and red hydrogen atoms). (Credit: Thiago Pinheiro dos Santos/Rice University)

https://news-network.rice.edu/news/files/2022/11/1124_NMR-2-web.jpg
Simulations by Rice University engineers revealed details about the magnetic interactions between gadolinium contrast agents used in MRI scans and their environment. From left: Philip Singer, Walter Chapman and Dilip Asthagiri. (Credit: Jeff Fitlow/Rice University)

https://news-network.rice.edu/news/files/2022/11/1124_NMR-3-web.jpg
Rice University graduate student Thiago Pinheiro dos Santos is lead author of a study that adds detail to models of gadolinium-based contrast agents used in MRI. (Credit: Rice University)
- Related materials
-
Modern simulations could improve MRIs: https://news2.rice.edu/2021/09/20/modern-simulations-could-improve-mris/
Chapman Research Group: https://www.ruf.rice.edu/~saft/
Chemical and Biomolecular Engineering: https://chbe.rice.edu
George R. Brown School of Engineering: https://engineering.rice.edu
- About Rice
-
Located on a 300-acre forested campus in Houston, Rice University is consistently ranked among the nation’s top 20 universities by U.S. News & World Report. Rice has highly respected schools of Architecture, Business, Continuing Studies, Engineering, Humanities, Music, Natural Sciences and Social Sciences and is home to the Baker Institute for Public Policy. With 4,240 undergraduates and 3,972 graduate students, Rice’s undergraduate student-to-faculty ratio is just under 6-to-1. Its residential college system builds close-knit communities and lifelong friendships, just one reason why Rice is ranked No. 1 for lots of race/class interaction and No. 1 for quality of life by the Princeton Review. Rice is also rated as a best value among private universities by Kiplinger’s Personal Finance.