HOUSTON – (March 7, 2022) – A surface that feels smooth to human touch could be pretty rough to a protein. That can be good or bad, depending on what you want that protein to do.

Exactly how proteins interact with solid surfaces is a concern for health care manufacturers who design drugs, make biosensors or develop anti-fouling materials.
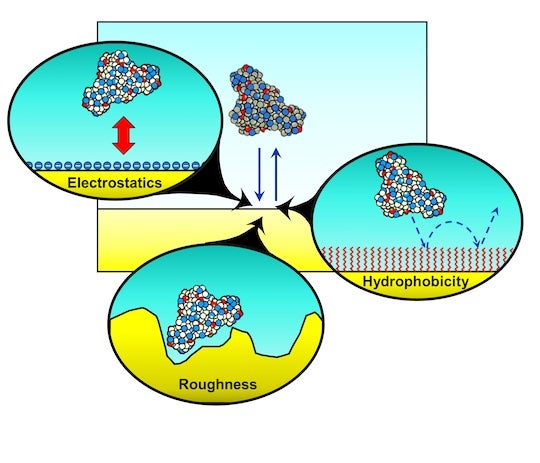
The mechanisms that control these interactions are hard to see, but researchers at Rice University are changing that with a microscopy technique to assess the effects of surface roughness as well as water-repelling properties (hydrophobicity) and electrostatic charge. The ability to tune those parameters will lead to more predictable materials.
“The main idea is to understand the how the combination of these properties influences protein dynamics,” said Anastasiia Misiura, lead author of a study in the Journal of Chemical Physics and a graduate student in the Rice lab of chemist Christy Landes. “It turned out that roughness and hydrophobicity are opposite forces, but proteins get stuck on areas that are very rough.”
The paper, an “editor’s choice,” is part of the journal’s “Ever-Expanding Optics of Single Molecules and Nanoparticles” collection.
How molecules interact at surfaces is important at every scale in the physical realm, from grinding planetary plates to brakes grabbing the wheels in your car to the invisible molecular transactions that make life possible. Understanding these mechanisms at the very smallest level is the focus of Landes’ lab as its members attempt to clarify what’s actually happening down there.
To that end, the lab develops sophisticated microscopes that see things smaller than visible light and the best of lenses will allow. In this case, the lab used single molecule fluorescence microscopy, a technique that allows them to watch how proteins interact with the surfaces they design.
The team discovered two modes of transport that influence whether and how proteins attach themselves to a surface, travel along it or release their grip, never to return. The two distinct interaction mechanisms they found ranged from the quicker localized adsorption/desorption, associated with less hydrophobic surfaces, and an unpredictable continuous-time random walk observed in interactions with rough, more hydrophobic surfaces.
For experiments, the researcher placed a “well-studied model protein,” fluorescent-labeled a-lactalbumin, on a surface with bare glass alternating with stripes in various concentrations of a self-assembled monolayer (SAM) commonly used to purify proteins via chromatography. Each stripe contained a different balance between hydrophobicity and surface roughness.

The bare glass showed plenty of localized action with proteins taking a longer time on the surface, while the degree of roughness in the SAM-covered regions (due to the concentration of octadecyltrichlorosilane, or ODTS) promoted longer flights. The degree of “stickiness” is associated with a greater concentration of long alkyl chains on the surface.
Understanding how to tailor surfaces could give manufacturers a handle to fine-tune protein interactions in their products, Landes said.
“Because all these complicated things are happening at different time scales and space scales, you could never separate the mechanistic contributions of each one of those individual effects,” she said. “The real value of single molecule spectroscopy and measuring at these scales is that you can distinguish the separate contributing factors.”
Co-authors are Rice postdoctoral researcher Chayan Dutta, graduate students Wesley Leung, Jorge Zepeda O and research scientist Tanguy Terlier. Landes is the Kenneth S. Pitzer Schlumberger Chair at Rice and a professor of chemistry, electrical and computer engineering and chemical and biomolecular engineering.
The Welch Foundation (C-1787) and the National Science Foundation (1808382, 1626418) supported the research.
- Peer-reviewed paper
-
“The competing influence of surface roughness, hydrophobicity, and electrostatics on protein dynamics on a self-assembled monolayer,” Journal of Chemical Physics
- Video
-

https://youtu.be/CUhdbVeeeGQ
Through single molecule fluorescence microscopy, researchers at Rice University were able to watch the dynamics of individual proteins as they interacted with smooth and rough surfaces, both of which are represented on this glass slide. The rough areas were more prone to hold proteins longer, revealing the possibility of tuning surfaces for such interactions. (Credit: Landes Research Group/Rice University) - Image downloads
-

https://news-network.rice.edu/news/files/2022/02/0228_ROUGH-1-WEB.jpg
Chemist Christy Landes, left, and graduate student Anastasiia Misiura set up an experiment in Landes’ Rice University laboratory. Using sophisticated microscopy techniques, their study shows why proteins stick better to some surfaces than others. The details could be important to manufacturers fine-tuning drug purification, biosensors or anti-fouling surfaces. (Credit: Jeff Fitlow/Rice University)
https://news-network.rice.edu/news/files/2022/02/0228_ROUGH-2-web.jpg
The influence of hydrophobicity, surface roughness and electrostatic effects on protein dynamics were the focus of a Rice University study. The details could be important to manufacturers fine-tuning drug purification, biosensors or anti-fouling surfaces. (Credit: Landes Research Group/Rice University)
https://news-network.rice.edu/news/files/2022/02/0228_ROUGH-3-web.jpg
Glass slides were patterned with self-assembled monolayers with varying degrees of a chemical known as ODTS to provide roughness for experiments to learn fine details of proteins’ dynamic interactions with surfaces. The Rice University study revealed mechanisms that could help manufacturers fine tune products used in drug design or as biosensors or anti-fouling surfaces. (Credit: Landes Research Group/Rice University) - Related materials and stories
-
Shaping light lets 2D microscopes capture 4D data: https://news2.rice.edu/2019/02/14/shaping-light-lets-2d-microscopes-capture-4d-data-2/
Landes Research Group: lrg.rice.edu/index.html
Department of Chemistry: chemistry.rice.edu
Wiess School of Natural Sciences: naturalsciences.rice.edu
Follow Rice News and Media Relations via Twitter @RiceUNews
- About Rice
-
Located on a 300-acre forested campus in Houston, Rice University is consistently ranked among the nation’s top 20 universities by U.S. News & World Report. Rice has highly respected schools of Architecture, Business, Continuing Studies, Engineering, Humanities, Music, Natural Sciences and Social Sciences and is home to the Baker Institute for Public Policy. With 4,052 undergraduates and 3,484 graduate students, Rice’s undergraduate student-to-faculty ratio is just under 6-to-1. Its residential college system builds close-knit communities and lifelong friendships, just one reason why Rice is ranked No. 1 for lots of race/class interaction and No. 1 for quality of life by the Princeton Review. Rice is also rated as a best value among private universities by Kiplinger’s Personal Finance.

