Rice University bioengineers are fabricating and testing tunable electrospun scaffolds completely derived from decellularized skeletal muscle to promote the regeneration of injured skeletal muscle.
Their paper in Science Advances shows how natural extracellular matrix can be made to mimic native skeletal muscle and direct the alignment, growth and differentiation of myotubes, one of the building blocks of skeletal muscle. The bioactive scaffolds are made in the lab via electrospinning, a high-throughput process that can produce single micron-scale fibers.
The research could ease the burden of performing an estimated 4.5 million reconstructive surgeries per year to repair injuries suffered by civilians and military personnel.
Current methods of electrospinning decellularized muscle require a copolymer to aid in scaffold fabrication. The Rice process does not.
“The major innovation is the ability to prepare scaffolds that are 100% extracellular matrix,” said bioengineer and principal investigator Antonios Mikos of Rice’s Brown School of Engineering. “That's very important because the matrix includes all the signaling motifs that are important for the formation of the particular tissue.”
The scaffolds leverage bioactive cues from decellularized muscle with the tunable material properties afforded through electrospinning to create a material rich with biochemical signals and highly specific topography. The material is designed to degrade as it is replaced by new muscle within the body.
Experiments revealed that cells proliferate best when the scaffolds are not saturated with a crosslinking agent, allowing them access to the biochemical cues within the scaffold matrix.
Electrospinning allowed the researchers to modulate crosslink density. They found that intermediate crosslinking led to better retention of fiber alignment during cell culture.
Most decellularized matrix for muscle regeneration comes from such thin membranes as skin or small intestine tissue. “But for muscle, because it’s thick and more complex, you have to cut it smaller than clinically relevant sizes and the original material properties are lost,” said Rice graduate student and lead author Mollie Smoak. “It doesn’t resemble the original material by the time you’re done.
“In our case, electrospinning was the key to make this material very tunable and have it resemble what it once was,” she said.
“It can generate fibers that are highly aligned, very similar to the architecture that one finds in skeletal muscle, and with all the biochemical cues needed to facilitate the creation of viable muscle tissue,” Mikos said.
Mikos said using natural materials rather than synthetic is important for another reason. “The presence of a synthetic material, and especially the degradation products, may have an adverse effect on the quality of tissue that is eventually formed,” he said.
“For eventual clinical application, we may use a skeletal muscle or matrix from an appropriate source because we're able to very efficiently remove the DNA that may elicit an immune response," Mikos said. "We believe that may make it suitable to translate the technology for humans.”
Smoak said the electrospinning process can produce muscle scaffolds in any size, limited only by the machinery.
“We’re fortunate to collaborate with a number of surgeons, and they see promise in this material being used for craniofacial muscle applications in addition to sports- or trauma-induced injuries to large muscles,” she said. “These would include the animation muscles in your face that are very fine and have very precise architectures and allow for things like facial expressions and chewing.”
Co-authors of the paper are Rice graduate student Katie Hogan and Jane Grande-Allen, the Isabel C. Cameron Professor of Bioengineering. Mikos is the Louis Calder Professor of Bioengineering and Chemical and Biomolecular Engineering.
The National Institutes of Health, the National Science Foundation and the Ford Foundation supported the research.
-30-
Read the abstract at https://advances.sciencemag.org/lookup/doi/10.1126/sciadv.abg4123.
Follow Rice News and Media Relations via Twitter @RiceUNews.
Related materials:
Grooves hold promise for sophisticated healing: https://news.rice.edu/2020/02/04/grooves-hold-promise-for-sophisticated-healing-2/
Molecular bait can help hydrogels heal wounds: https://news.rice.edu/2019/06/05/molecular-bait-can-help-hydrogels-heal-wounds-2/
Mikos Research Group: https://www.ruf.rice.edu/~mikosgrp/
Biomaterials Lab: bml.rice.edu
Rice Department of Bioengineering: https://bioengineering.rice.edu
George R. Brown School of Engineering: https://engineering.rice.edu
Images for download:
https://news2.rice.edu/files/2021/04/0405_SCAFFOLDS-1-WEB.jpg
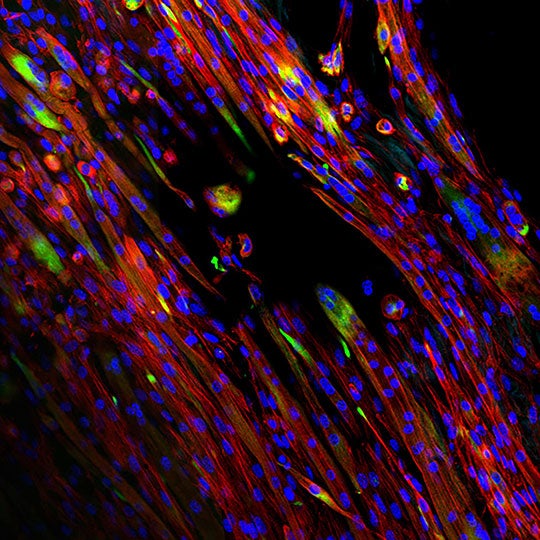
Aligned myotubes formed on electrospun extracellular matrix scaffolds produced at Rice University. The staining with fluorescent tags shows cells’ expression of myogenic marker desmin (green), actin (red) and nuclei (blue) after seven days of growth. (Credit: Mikos Research Group/Rice University)
https://news2.rice.edu/files/2021/04/0405_SCAFFOLDS-2-WEB.jpg
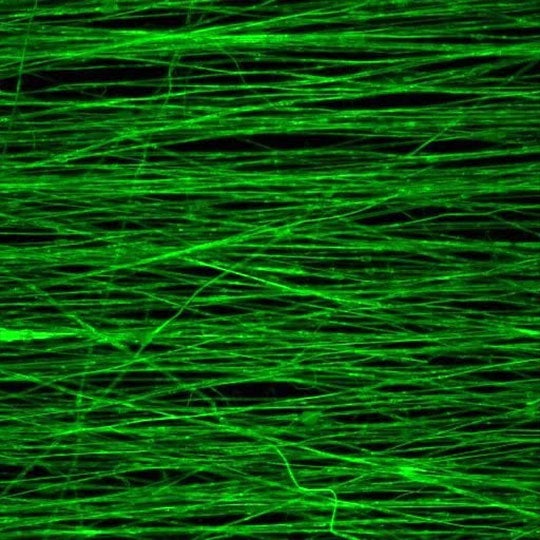
Aligned fibers produced via electrospinning can be used to form a tunable scaffold for growing new muscle, according to Rice University bioengineers. These fibers were fabricated with decellularized skeletal muscle extracellular matrix on a mandrel spinning at 3,000 rotations per minute.
https://news2.rice.edu/files/2021/04/0405_SCAFFOLDS-3-WEB.jpg
Samples of electrospun decellularized skeletal muscle extracellular matrix created by Rice University bioengineers. Such scaffolds can be used to regenerate injured muscles. The natural scaffold material degrades as new muscles take over. (Credit: Mikos Research Group/Rice University)
https://news2.rice.edu/files/2021/04/0405_SCAFFOLDS-4-WEB.jpg
A sample of decellularized extracellular matrix created by Rice University bioengineers. The electrospun scaffolds can be used to regenerate injured muscles. The natural scaffold material degrades as new muscles take over.
https://news2.rice.edu/files/2021/04/0405_SCAFFOLDS-5-WEB.jpg
Rice University graduate students Katie Hogan, left, and Mollie Smoak prepare to fabricate a scaffold with an electrospinner. The scaffolds derived from decellularized skeletal muscle are designed to promote regeneration of injured skeletal muscle. (Credit: Jeff Fitlow/Rice University)
Located on a 300-acre forested campus in Houston, Rice University is consistently ranked among the nation’s top 20 universities by U.S. News & World Report. Rice has highly respected schools of Architecture, Business, Continuing Studies, Engineering, Humanities, Music, Natural Sciences and Social Sciences and is home to the Baker Institute for Public Policy. With 3,978 undergraduates and 3,192 graduate students, Rice’s undergraduate student-to-faculty ratio is just under 6-to-1. Its residential college system builds close-knit communities and lifelong friendships, just one reason why Rice is ranked No. 1 for lots of race/class interaction and No. 1 for quality of life by the Princeton Review. Rice is also rated as a best value among private universities by Kiplinger’s Personal Finance.