HOUSTON – (July 14, 2020) – Where things get sticky happens to be where interesting science happens in a Rice University lab working to improve battery technology.
Using techniques similar to those they employed to develop laser-induced graphene, Rice chemist James Tour and his colleagues turned adhesive tape into a silicon oxide film that replaces troublesome anodes in lithium metal batteries.
For the Advanced Materials study, the researchers used an infrared laser cutter to convert the silicone-based adhesive of commercial tape into the porous silicon oxide coating, mixed with a small amount of laser-induced graphene from the tape’s polyimide backing. The protective silicon oxide layer forms directly on the current collector of the battery.
The idea of using tape came from previous attempts to produce free-standing films of laser-induced graphene, Tour said. Unlike pure polyimide films, the tape produced not only laser-induced graphene from the polyimide backing but also a translucent film where the adhesive had been. That caught the curiosity of the researchers and led to further experimentation.
The layer formed when they stuck the tape to a copper current collector and lased it multiple times to quickly raise its temperature to 2,300 Kelvin (3,680 degrees Fahrenheit). That generated a porous coating composed primarily of silicon and oxygen, combined with a small amount of carbon in the form of graphene.
In experiments, the foamy film appeared to soak up and release lithium metal without allowing the formation of dendrites -- spiky protrusions -- that can short-circuit a battery and potentially cause fires. The researchers noted lithium metal tends to degrade fast during the battery’s charge and discharge cycles with the bare current collector, but no such problems were observed in anodes coated with laser-induced silicon oxide (LI-SiO).
“In traditional lithium-ion batteries, lithium ions are intercalated into a graphite structure upon charging and de-intercalate as the battery discharges,” said lead author Weiyin Chen, a Rice graduate student. “Six carbon atoms are used to store one lithium atom when the full capacity of graphite is used.
“But in a lithium metal anode, no graphite is used,” he said. “The lithium ions directly shuttle from the surface of the metal anode as the battery discharges. Lithium metal anodes are considered a key technology for future battery development once their safety and performance issues are solved.”
Lithium metal anodes can have a capacity 10 times higher than traditional graphite-lithium ion batteries. But lithium metal batteries that are devoid of graphite usually use excess lithium metal to compensate for losses caused by oxidation of the anode surface, Tour said.
“When there is zero excess lithium metal in the anodes, they generally suffer fast degradation, producing cells with very limited cycle life,” said co-author Rodrigo Salvatierra, an academic visitor in the Tour lab. “On the bright side, these ‘anode-free’ cells become lighter and deliver better performance, but with the cost of a short life.”
The researchers noted LI-SiO tripled the battery lifetimes over other zero-excess lithium metal batteries. The LI-SiO coated batteries delivered 60 charge-discharge cycles while retaining 70% of their capacity.
Tour said that could make lithium metal batteries suitable as high-performance batteries for outdoor expeditions or high-capacity storage for short-term outages in rural areas.
Using standard industrial lasers should allow industry to scale up for large-area production. Tour said the method is fast, requires no solvents and can be done in room atmosphere and temperature. He said the technique may also produce films to support metal nanoparticles, protective coatings and filters.
Co-authors are graduate students Muqing Ren and Jinhang Chen and postdoctoral researcher Michael Stanford. Tour is the T.T. and W.F. Chao Chair in Chemistry as well as a professor of computer science and of materials science and nanoengineering at Rice.
The Air Force Office of Scientific Research supported the project.
-30-
Read the abstract at https://onlinelibrary.wiley.com/doi/10.1002/adma.202002850.
Follow Rice News and Media Relations via Twitter @RiceUNews.
Related materials:
Better red than dread: Barrier keeps batteries safe: http://news.rice.edu/2019/02/14/better-red-than-dread-barrier-keeps-batteries-safe-2/
Tour Group at Rice: https://www.jmtour.com
Department of Chemistry: https://chemistry.rice.edu
Wiess School of Natural Sciences: https://naturalsciences.rice.edu
Image for download:
https://news-network.rice.edu/news/files/2020/07/0720_ADHESIVE-1-WEB.jpg
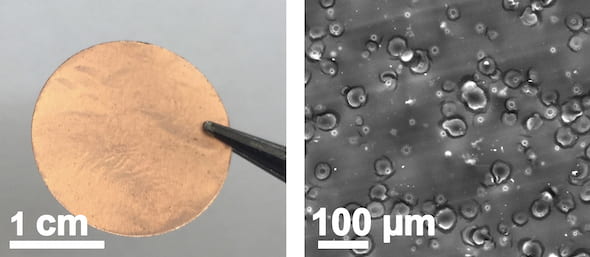
At left, a copper current collector with a laser-induced silicon oxide coating created at Rice University. At right, a scanning electron microscope image of the coating created by lasing adhesive tape on the copper collector. (Credit: Tour Group/Rice University)
Located on a 300-acre forested campus in Houston, Rice University is consistently ranked among the nation’s top 20 universities by U.S. News & World Report. Rice has highly respected schools of Architecture, Business, Continuing Studies, Engineering, Humanities, Music, Natural Sciences and Social Sciences and is home to the Baker Institute for Public Policy. With 3,962 undergraduates and 3,027 graduate students, Rice’s undergraduate student-to-faculty ratio is just under 6-to-1. Its residential college system builds close-knit communities and lifelong friendships, just one reason why Rice is ranked No. 1 for lots of race/class interaction and No. 4 for quality of life by the Princeton Review. Rice is also rated as a best value among private universities by Kiplinger’s Personal Finance.