HOUSTON – (Jan. 29, 2020) – The next time you’re thinking about whether to cook dinner or order a pizza for delivery, think of this: Plants have been doing pretty much the same thing for eons.
Researchers in Rice University's Systems, Synthetic and Physical Biology program detailed how plants have evolved to call for nutrients, using convenient bacteria as a delivery service.
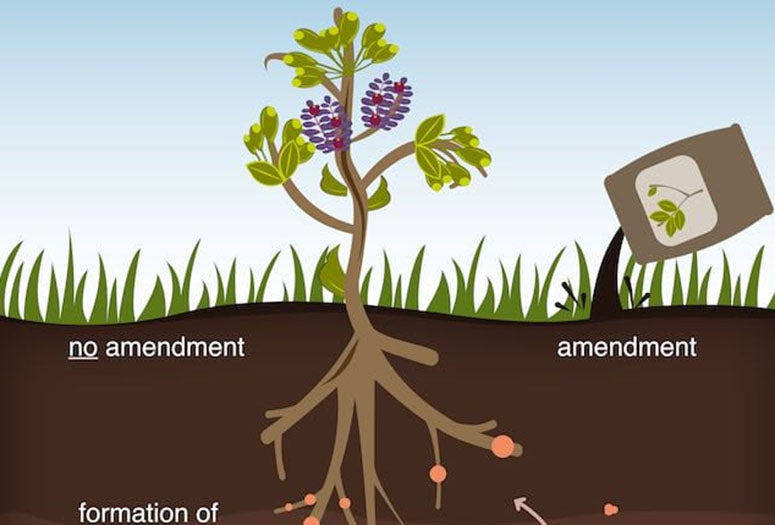
Their open-access report in Science Advances looks at how plants read the local environment and, when necessary, make and release molecules called flavonoids. These molecules attract microbes that infect the plants and form nitrogen nodules -- where food is generated -- at their roots.
When nitrogen is present and available, plants don't need to order in. Their ability to sense the presence of a nearby slow-release nitrogen source, organic carbon, is the key.
"It’s a gorgeous example of evolution: Plants change a couple of (oxygen/hydrogen) groups here and there in the flavonoid, and this allows them to use soil conditions to control which microbes they talk to," said Rice biogeochemist Caroline Masiello, a co-author of the study.
The Rice team, in collaboration with researchers at Cornell University, specifically analyzed how flavonoids mediate interactions between plants and microbes depending on the presence of abiotic (nonliving) carbon. Their experiments revealed, to their surprise, that an excess of dissolved -- rather than solid -- carbon in soil effectively quenches flavonoid signals.
Understanding how carbon in soil affects these signals may provide a way to engineer beneficial interactions between plants and microbes and to design effective soil amendments (additives that balance deficiencies in soil), according to the researchers. Plants use flavonoids as a defense mechanism against root pathogens and could manipulate the organic carbon they produce to interfere with signaling between microbes and other plants that compete for the same nutrients.
Overall, they showed that higher organic carbon levels in soil repressed flavonoid signals by up to 98%. In one set of experiments, interrupting the signals between legume plants and microbes sharply cut the formation of nitrogen nodules.
Rice graduate student Ilenne Del Valle began the study when she became interested in the subtle differences between the thousands of flavonoids and how they influence connections between plants and microbes in soil.
"We had studied how different soil amendments change how microbes communicate with one another," said Del Valle, co-lead author of the paper with former Cornell postdoctoral associate Tara Webster. "The next question was whether this was happening when the microbes communicate with plants.
"We knew that plants modulate symbiosis with microbes through flavonoid molecules," she said. "So we wanted to learn how flavonoids interact with soil amendments used for different purposes in agriculture."
Because she counts two Rice professors -- Masiello and synthetic biologist Joff Silberg -- as her advisers, she had access to tools from both disciplines to discover the mechanisms behind those subtleties.
"We came into this thinking there was going to be a big effect from biochar," Silberg said. "Biochar is charcoal made for agricultural amendment, and it is well-known to affect microbe-microbe signals. It has a lot of surface area, and flavonoids look sticky, too. People thought they would stick to the biochar.
"They didn't. Instead, we found that dissolved carbon moving through water in the soil was affecting signals," he said. "It was very different from all of our expectations."
The Rice and Cornell team set up experiments with soils from meadows, farms and forests and then mixed in three slightly different flavonoids: naringenin, quercetin and luteolin.
They found the most dramatic effects when dissolved carbons derived from plant matter or compost were present. Plants employ naringenin, a variant of the flavonoid that gives grapefruit its bitter taste, and luteolin, expressed by leaves and many vegetables, to call for microbial nitrogen fixation. These were most curtailed in their ability to find microbes. Quercetin, also found in foods like kale and red onions and used for defense against pests, did not suffer the same fate.
Masiello noted there's a cost for plants to connect with microbes in the soil.
"These relationships with symbionts are metabolically costly," she said. "Plants have to pay the microbes in photosynthesized sugar, and in exchange the microbes mine the soil for nutrients. Microbial symbionts can be really expensive subcontractors, sometimes taking a significant fraction of a plant’s photosynthate.
"What Ilenne and Tara have shown is one mechanism through which plants can control whether they invest in expensive symbionts," she said. "Among a wide class of signaling compounds used by plants for many purposes, one specific signal related to nutrients is shut off by high soil organic matter, which is a slow-release source of nutrients. The plant signal that says 'come live with us' doesn’t get through.
"This is good for plants because it means they don’t waste photosynthate supporting microbial help they don’t need. Ilenne and Tara have also shown that signals used for other purposes are slightly chemically modified so their transmission is not affected at the same rate."
The researchers checked flavonoid concentrations in soil with standard chromatography as well as unique fluorescent and gas biosensors, genetically modified microbes introduced in 2016 with the support of a Keck Foundation grant, which also backed the current project. The microbes release a gas when they sense a particular microbial interaction in opaque materials like soil.
"The gas sensor ended up being very useful in experiments that looked like tea, where we couldn't image fluorescent signals," Silberg said.
Co-authors of the paper are Rice alumna Hsiao-Ying Cheng, graduate student Mary Kaitlyn Miller and Zachary Ball, an associate professor of chemistry; Janice Thies, an associate professor of soil chemistry, André Kessler, an assistant professor of ecology and evolutionary biology, and Johannes Lehmann, the Liberty Hyde Bailey professor of soil biogeochemistry and soil fertility management, all at Cornell; and Kevin MacKenzie, an associate professor of pathology and pharmacology at Baylor College of Medicine and an adjunct professor at Rice.
Masiello is a professor of Earth, environmental and planetary sciences at Rice. Silberg is the Stewart Memorial Professor of BioSences.
The Taiwan Ministry of Education Scholarship, the National Science Foundation, the Department of Energy Office of Science and the U.S. Department of Agriculture also supported the research.
-30-
Read the paper at https://advances.sciencemag.org/content/6/5/eaax8254
Follow Rice News and Media Relations via Twitter @RiceUNews.
Related materials:
Biochar quiets microbes, including some plant pathogens: https://news.rice.edu/2013/09/30/biochar-quiets-microbes-including-some-plant-pathogens/
Gas sensors 'see' through soil to analyze microbial interactions: http://news.rice.edu/2016/07/18/gas-sensors-see-through-soil-to-analyze-microbial-interactions-2/
Masiello Lab: https://www.masiellolab.org
Silberg Lab: https://www.silberglab.org
Rice Department of BioSciences: https://biosciences.rice.edu
Rice Department of Earth, Environmental and Planetary Sciences: https://earthscience.rice.edu
Systems, Synthetic and Physical Biology program: https://sspb.rice.edu/
Images for download:
https://news-network.rice.edu/news/files/2019/10/1021_SOIL-1E-WEB-2.jpg
(Credit: Illustration by Ilenne Del Valle/Rice University)
https://news-network.rice.edu/news/files/2019/10/1021_SOIL-2E-WEB-2.jpg
(Credit: Illustration by Ilenne Del Valle/Rice University)
https://news-network.rice.edu/news/files/2019/10/1021_SOIL-7-WEB.jpg
Rice University graduate student Ilenne Del Valle used unique fluorescent and gas biosensors to learn new details about how plants read the environment around them and communicate with nearby microbes to bring them nutrients. (Credit: Jeff Fitlow/Rice University)
https://news-network.rice.edu/news/files/2019/10/1021_SOIL-4-WEB-2.jpg
Rice University graduate student Ilenne Del Valle holds samples of soil with various concentrations of organic carbon and proteins produced by plants that regulate the acquisition of nutrients and pest control. (Credit: Jeff Fitlow/Rice University)
https://news-network.rice.edu/news/files/2019/10/1021_SOIL-5-WEB.jpg
Rice University researchers used a trio of soil amendments to test the process by which plants communicate with microbes to obtain nutrients and ward off pests. Clockwise from top left are mesquite chips, maple chips and charcoal made from maple chips. (Credit: Jeff Fitlow/Rice University)
https://news-network.rice.edu/news/files/2019/10/1021_SOIL-6-WEB.jpg
Rice University graduate student Ilenne Del Valle, left, and biogeochemist Caroline Masiello oversee an experiment during their study of how plants communicate with microbes in soil and how organic carbon in soil can block signals in ways that ultimately benefit plants. (Credit: Jeff Fitlow/Rice University)
Located on a 300-acre forested campus in Houston, Rice University is consistently ranked among the nation’s top 20 universities by U.S. News & World Report. Rice has highly respected schools of Architecture, Business, Continuing Studies, Engineering, Humanities, Music, Natural Sciences and Social Sciences and is home to the Baker Institute for Public Policy. With 3,962 undergraduates and 3,027 graduate students, Rice’s undergraduate student-to-faculty ratio is just under 6-to-1. Its residential college system builds close-knit communities and lifelong friendships, just one reason why Rice is ranked No. 1 for lots of race/class interaction and No. 4 for quality of life by the Princeton Review. Rice is also rated as a best value among private universities by Kiplinger’s Personal Finance.