Rice University, Rensselaer study reveals graphene enhances many materials, but leaves them wettable
Graphene is largely transparent to the eye and, as it turns out, largely transparent to water.
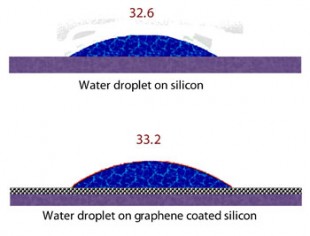
A new study by scientists at Rice University and Rensselaer Polytechnic Institute (RPI) has determined that gold, copper and silicon get just as wet when clad by a single continuous layer of graphene as they would without.
 The research, reported this week in the online edition of Nature Materials, is significant for scientists learning to fine-tune surface coatings for a variety of applications.
The research, reported this week in the online edition of Nature Materials, is significant for scientists learning to fine-tune surface coatings for a variety of applications.
“The extreme thinness of graphene makes it a totally noninvasive coating,” said Pulickel Ajayan, Rice’s Benjamin M. and Mary Greenwood Anderson Professor in Mechanical Engineering and Materials Science and of chemistry. “A drop of water sitting on a surface ‘sees through’ the graphene layers and conforms to the wetting forces dictated by the surface beneath. It’s quite an interesting phenomenon unseen in any other coatings and once again proves that graphene is really unique in many different ways.” Ajayan is co-principal investigator of the study with Nikhil Koratkar, a professor of mechanical, aerospace and nuclear engineering at RPI.
A typical surface of graphite, the form of carbon most commonly known as pencil lead, should be hydrophobic, Ajayan said. But in the present study, the researchers found to their surprise that a single-atom-thick layer of the carbon lattice presents a negligible barrier between water and a hydrophilic – water-loving – surface. Piling on more layers reduces wetting; at about six layers, graphene essentially becomes graphite.
An interesting aspect of the study, Ajayan said, may be the ability to change such surface properties as conductivity while retaining wetting characteristics. Because pure graphene is highly conductive, the discovery could lead to a new class of conductive, yet impermeable, surface coatings, he said.
The caveat is that wetting transparency was observed only on surfaces (most metals and silicon) where interaction with water is dominated by weak van der Waals forces, and not for materials like glass, where wettability is dominated by strong chemical bonding, the team reported.
But such applications as condensation heat transfer — integral to heating, cooling, dehumidifying, water harvesting and many industrial processes — may benefit greatly from the discovery, according to the paper. Copper is commonly used for its high thermal conductivity, but it corrodes easily. The team coated a copper sample with a single layer of graphene and found the subnanometer barrier protected the copper from oxidation with no impact on its interaction with water; in fact, it enhanced the copper’s thermal effectiveness by 30 to 40 percent.
“The finding is interesting from a fundamental point of view as well as for practical uses,” Ajayan said. “Graphene could be one of a kind as a coating, allowing the intrinsic physical nature of surfaces, such as wetting and optical properties, to be retained while altering other specific functionalities like conductivity.”
The paper’s co-authors are Rice graduate student Hemtej Gullapalli, RPI graduate students Javad Rafiee, Xi Mi, Abhay Thomas and Fazel Yavari, and Yunfeng Shi, an assistant professor of materials science and engineering at RPI.
The Advanced Energy Consortium, National Science Foundation and the Office of Naval Research graphene MURI program funded the research.


Leave a Reply