HOUSTON – (Jan. 14, 2020) – Intentional defects in batteries have given Rice University scientists a window into the hazards of pushing lithium-ion cells too far.
New simulations by Rice materials scientist Ming Tang and graduate student Kaiqi Yang, detailed in the Journal of Materials Chemistry A, shows too much stress in widely used lithium iron phosphate cathodes can open cracks and quickly degrade batteries.
The work extends recent Rice research that demonstrated how putting defects in particles that make up the cathode could improve battery performance by up to two orders of magnitude by helping lithium move more efficiently.
But the lab’s subsequent modeling study revealed a caveat. Under the pressure of rapid charging and discharging, defect-laden cathodes risk fracture.
“The conventional picture is that lithium moves uniformly into the cathode, with a lithium-rich region that expands smoothly into the cathode’s center,” said Tang, an assistant professor of materials science and nanoengineering at Rice’s Brown School of Engineering.
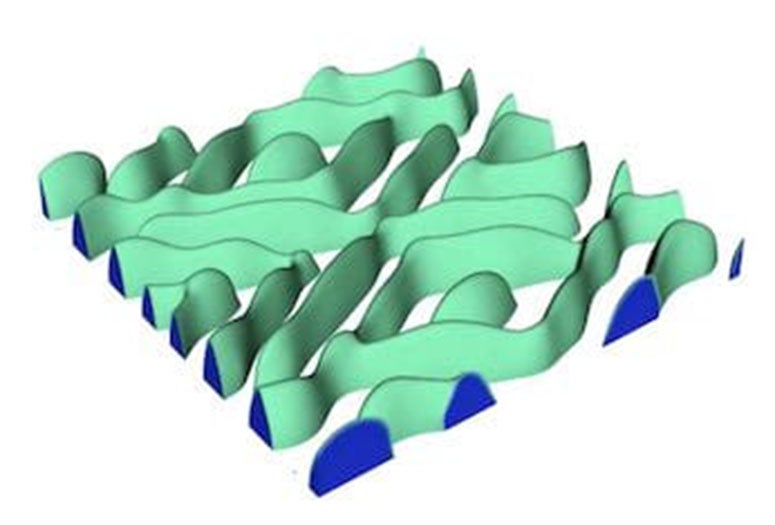
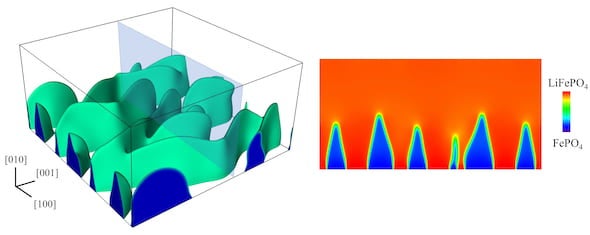
But X-ray images taken at another lab showed something else. “They saw a fingerlike boundary between the lithium-rich and lithium-poor regions, almost like when you inject water into oil,” he said. “Our question was, what causes this?"
The root of the problem appears to be that stress destabilizes the initially flat boundary and causes it to become wavy, Tang said. The change in the boundary shape further increases the stress level and triggers crack formation. The study by Tang’s group shows that such instability can be increased by a common type of defect in battery compounds called antisites, where iron atoms occupy spots in the crystal where lithium atoms should be.
“Antisites can be a good thing, as we showed in the last paper, because they accelerate the lithium intercalation kinetics,” Tang said, “But here we show a countereffect: Too many antisites in the particles encourage the moving interface to become unstable and therefore generate more stress.”
Tang believes there’s a sweet spot for the number of antisites in a cathode: enough to enhance performance but too few to promote instability. “You want to have a suitable level of defects, and it will require some trial and error to figure out how to reach the right amount through annealing the particles,” he said. “We think our new predictions might be useful to experimentalists.”
The U.S. Department of Energy (DOE) supported the research. Simulations were performed on supercomputers at the Texas Advanced Computing Center at the University of Texas and DOE’s National Energy Research Scientific Computing Center.
-30-
Read the abstract at https://pubs.rsc.org/en/content/articlelanding/2020/ta/c9ta11697d#!divAbstract.
Follow Rice News and Media Relations via Twitter @RiceUNews.
Video:
A 3D model by Rice University materials scientists shows the phase evolution of a delithiating lithium iron phosphate cathode undergoing rapid discharge. The "fingerlike" shape adds stress to the system that researchers suspect can lead to cracks in the cathode that degrade the battery. (Credit: Mesoscale Materials Science Group/Rice University)
Images for download:
https://news-network.rice.edu/news/files/2020/01/0121_BATTERY-1-WEB.jpg
At left, a 3D model by Rice University materials scientists shows a phase boundary as a delithiating lithium iron phosphate cathode undergoes rapid discharge. At right, a cross-section shows the “fingerlike” boundary between iron phosphate (blue) and lithium (red). Rice engineers found that too many intentional defects intended to make batteries better can in fact degrade their performance and endurance. (Credit: Mesoscale Materials Science Group/Rice University)
https://news-network.rice.edu/news/files/2020/01/0121_BATTERY-2-WEB.jpg
Rice graduate student Kaiqi Yang, left, and materials scientist Ming Tang determined that the fast charge and discharge of some lithium-ion batteries with intentional defects degrades their performance and endurance. (Credit: Jeff Fitlow/Rice University)
Related materials:
Detours may make batteries better: http://news.rice.edu/2019/12/09/detours-may-make-batteries-better-2/
Mesoscale Materials Science Group: http://tanggroup.rice.edu/research/
Department of Materials Science and NanoEngineering: https://msne.rice.edu
George R. Brown School of Engineering: https://engineering.rice.edu
Located on a 300-acre forested campus in Houston, Rice University is consistently ranked among the nation’s top 20 universities by U.S. News & World Report. Rice has highly respected schools of Architecture, Business, Continuing Studies, Engineering, Humanities, Music, Natural Sciences and Social Sciences and is home to the Baker Institute for Public Policy. With 3,962 undergraduates and 3,027 graduate students, Rice’s undergraduate student-to-faculty ratio is just under 6-to-1. Its residential college system builds close-knit communities and lifelong friendships, just one reason why Rice is ranked No. 1 for lots of race/class interaction and No. 4 for quality of life by the Princeton Review. Rice is also rated as a best value among private universities by Kiplinger’s Personal Finance.