Pollution from ammonia-based fertilizer reverts back to ammonia — with a side of rocket fuel
Rice University researchers have identified a simpler way to rid water of cancer-causing pollutants and turn them into valuable chemicals.
Michael Wong, Thomas Senftle and their team have discovered a new catalyst that can turn nitrite pollutant waste into ammonia, a compound mostly used as fertilizer and household cleaner, as well as hydrazine, which is used as rocket fuel.
“Agricultural fertilizer runoff is contaminating ground and surface water, which causes ecological effects such as algae blooms as well as significant adverse effects for humans, including cancer, hypertension and developmental issues in babies,” said Wong, professor and chair of the Department of Chemical and Biomolecular Engineering in Rice’s Brown School of Engineering. “I’ve been very curious about nitrogen chemistry, especially if I can design materials that clean water of nitrogen compounds like nitrites and nitrates.”
The study, published in the journal ACS Catalysis, challenges the idea that only palladium-based catalysts are effective for nitrite reduction and expands the frontiers of the reduction process.
The creation of ammonia from nitrite waste is especially exciting to Wong’s team. Ammonia-based fertilizers are critical for global food supplies, and ammonia has also been discussed as a carbon-free liquid fuel that could address climate change. But ammonia producers still rely heavily on the energy-intensive Haber-Bosch process, and making ammonia from nitrite waste could provide a green alternative, Wong said.
Over the past two decades, removing water contaminants through catalysis has garnered attention as a promising technology. Because palladium is regarded as the most effective metal for nitrate and nitrite elimination, few studies have thoroughly explored the performance of other metal catalysts.
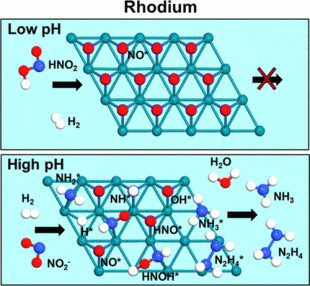
At higher pH values, rhodium created significant quantities of ammonium and smaller amounts of hydrazine.
Wong’s team of students and collaborators tested how well a rhodium catalyst could remove nitrite compared to a well-known palladium material. The team concluded that at higher pH values, palladium created mostly dinitrogen while rhodium created significant quantities of ammonium and smaller amounts of hydrazine.
“What initially launched this project was the desire to find a cheaper metal than the commonly used palladium,” said Wong, who’s also a professor of chemistry and director of Rice’s Catalysis and Nanomaterials Laboratory. “While rhodium isn’t cheaper, we discovered that it can do something that palladium cannot — conduct the chemistry at a higher pH level and create much more ammonia. This was new chemistry, which sparked subsequent questions, and we just continued to follow the breadcrumbs.”
“The study explains why this material becomes more effective under conditions that would cause conventional catalysts to deactivate,” said Senftle, assistant professor of chemical and biomolecular engineering. “That opens new avenues for designing robust nitrite reduction catalysts.”
The paper builds on nitrogen chemistry research that’s increasingly become a focus for Wong and his team. In 2017, they published research on a material that could quickly and cheaply turn nitrate into dinitrogen gas.
Wong thinks catalytic converter technology based on the new rhodium catalyst could be most useful as a filter installed at sites prone to runoff, such as farms.
“The fact that we can start thinking about where to implement this is because we can now access the chemistry,” he said.
Wong and his team were most surprised by the rhodium catalyst’s creation of hydrazine.
“The thing that excites me most about this research is the observation of hydrazine,” said Chelsea Clark, a graduate student in Wong’s lab. “This gives us new ideas on how to make other useful chemicals from nitrite waste waters.”
While Wong and his team don’t yet know how this chemistry can best be implemented, they are eager to explore the opportunities it presents.
“I’m excited about removing nitrite, forming ammonia and hydrazine, as well as the chemistry that we figured out about how all this happens,” Wong said. “The most important takeaway is that we learned how to clean water in a simpler way and created chemicals that are more valuable than the waste stream.”
Additional co-authors include C. Prakash Reddy, Hao Xu, Kimberly Heck and Guohua Luo, all of Rice. The research was partially supported by the National Science Foundation’s Nanosystems Engineering Research Center for Nanotechnology Enabled Water Treatment (NEWT).


