by Kayt Sukel
Special to Rice News
Rice University researchers in the lab of chemist Han Xiao have identified a promising new immunological pathway to treat stubborn bone tumors, one of most prevalent forms of metastases in breast cancer patients.

“More than 70% of people with metastatic breast cancer will see the cancer cells move to bone, which can lead to skeletal-related events like bone pain, fractures, and hypercalcemia,” said Yixian Wang, a Rice graduate student in the Han lab who is a lead author on a study published in Proceedings of the National Academy of Sciences. “There are now several immunotherapies that can potentially benefit breast cancer patients with metastases, but they aren’t effective in patients with bone tumors.”
Each year, more than 240,000 new cases of breast cancer are diagnosed in the United States, according to the Centers for Disease Control and Prevention. Approximately one-quarter of those patients will experience metastasis, where cancer cells spread from the breast to other parts of the body. New immunotherapies called checkpoint inhibitors can uncloak stubborn tumors, allowing the immune system to send in powerful immune cells to deal with the abnormal cells. But while checkpoint inhibitors are effective for many patients, they do not work for everyone ⎯ and clinical trials have shown little to no response when used to treat bone metastases.
Xiao, associate professor of chemistry, biosciences, and bioengineering at Rice, said he and his team wanted to find another pathway that might be more effective in obliterating these stubborn bone metastases.
“We thought there must be another novel checkpoint axis we could target for the breast cancer cells in bone,” Xiao said. “And we discovered a unique glyco-immune checkpoint axis in bone metastases that involves a protein called sialic acid-binding Ig-like lectin (Siglec)-15. We learned that it suppresses immune cells in the bone.”
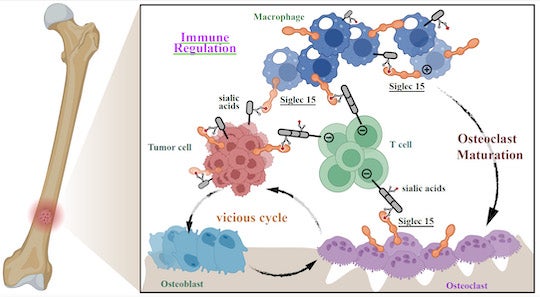
After noting that there was a significant upregulation of Siglec-15 in the tumor microenvironment in bone tumor samples from breast cancer patients, Xiao and colleagues demonstrated that this receptor plays an important role in hiding bone tumors from the immune surveillance.
“Current FDA-approved checkpoint inhibitors are mediated by protein-protein interactions that suppress immune cells,” Xiao explained. “Siglec-15, however, is a glyco-immune checkpoint inhibitor. Instead of binding to a protein, Siglec-15 binds to the sugars you find on the cell surfaces ⎯ and that’s how it can suppress the immune system. This is an entirely new type of immune checkpoint that offers great promise for future treatment for bone cancers.”
Xiao’s team conducted several cell culture experiments to study Siglec-15 interactions in the bone tumor microenvironment. They learned it is involved in crosstalk between tumor cells and important immune cells like T-cells and macrophages, as well as bone-specific cells, osteoclasts.
“You can find these glycolipids and glycoproteins on all cells ⎯ and we know they play an important role in immune modulation,” added Xiao. “These findings offer us an opportunity to study these glyco-immune checkpoint inhibitors more in depth and identify those that can help bone tumors stop evading immune recognition.”

But simply modulating the behavior of Siglec-15 may be enough to treat bone metastases. When the team injected a monoclonal antibody that targets Siglec-15 into an animal model of metastatic breast cancer with bone tumors, they were able to trigger a powerful immune response. In fact, the researchers saw the tumors diminish after only one or two doses of the antibody therapy.
“It was really a striking finding,” said Wang. “I’m very excited about the potential therapeutic outcome for a therapy like this. This could be a very helpful treatment for breast cancer patients in the future.”
Xiao said he and his team plan to continue studying the new and unique biology of glyco-immune checkpoint pathways in the tumor microenvironment. He added there is still much to learn about these pathways and future studies should provide new biological insights with the power to improve both current and future immunotherapies. In addition, Xiao said he would like to see if targeting Siglec-15 might be helpful in treating other kinds of cancers that affect bone.
The research was supported by the Cancer Prevention Research Institute of Texas (RR170014), the National Institutes of Health (R01-CA277838, R35-GM133706, R21-CA255894, R01-AI165079) the U.S. Department of Defense (HT9425-23-1-0494, W81XWH-21-1-0789), the John S. Dunn Foundation and Rice University.
- Peer-reviewed paper:
-
Siglec-15/Sialic Acid Axis as a Central Glyco-Immune Checkpoint in Breast Cancer Bone Metastasis | Proceedings of the National Academy of Sciences | DOI: 10.1073/pnas.2312929121
Authors: Yixian Wang, Zhan Xu, Kuan-Lin Wu, Liqun Yu, Chenhang Wang, Haoxue Ding, Yang Gao, Han Sun, Yi-Hsuan Wu, Meng Xia, Yuda Chen and Han Xiao
https://doi.org/10.1073/pnas.2312929121 - Image downloads:
-
https://news-network.rice.edu/news/files/2024/01/240103_Han-Xiao-with-Yixian-Wang_Gustavo--af8f3518d906de73.jpg
CAPTION: Han Xiao (left) and Yixian Wang (Photo by Gustavo Raskosky/Rice University)
https://news-network.rice.edu/news/files/2024/01/240103_Han-Xiao-with-Yixian-Wang_Gustavo-102207-4a7364784651b99c.jpg
CAPTION: Yixian Wang (left) and Han Xiao (Photo by Gustavo Raskosky/Rice University)
https://news-network.rice.edu/news/files/2024/01/research-schematic-f7a95407b821945f.jpg
CAPTION: Siglec-15 is involved in the crosstalk between metastatic bone tumors and immune cells, establishing a vicious cycle that allows immune cell suppression. (Image courtesy of the Xiao lab/Rice University)
- Related stories:
-
Rice U. chemist wins $3.2 million National Cancer Institute grant:
https://news.rice.edu/news/2023/rice-chemist-wins-32m-national-cancer-institute-grant
Rice, Baylor developing ‘glyco-immune’ checkpoint inhibitor:
https://news.rice.edu/news/2023/rice-baylor-developing-glyco-immune-checkpoint-inhibitorAntibody with engineered peptide targets bone metastasis:
https://news.rice.edu/news/2022/antibody-engineered-peptide-targets-bone-metastasis - Links:
-
The Xiao lab: https://xiao.rice.edu/People/hanxiao/hanxiao.html
Rice Department of Chemical and Biomolecular Engineering: https://chbe.rice.edu/
Rice Department of Chemistry: https://chemistry.rice.edu/
Wiess School of Natural Sciences: https://naturalsciences.rice.edu/George R. Brown School of Engineering: https://engineering.rice.edu
Bioscience Research Collaborative: https://brc.rice.edu/
- About Rice:
-
Located on a 300-acre forested campus in Houston, Rice University is consistently ranked among the nation’s top 20 universities by U.S. News & World Report. Rice has highly respected schools of architecture, business, continuing studies, engineering, humanities, music, natural sciences and social sciences and is home to the Baker Institute for Public Policy. With 4,574 undergraduates and 3,982 graduate students, Rice’s undergraduate student-to-faculty ratio is just under 6-to-1. Its residential college system builds close-knit communities and lifelong friendships, just one reason why Rice is ranked No. 1 for lots of race/class interaction, No. 2 for best-run colleges and No. 12 for quality of life by the Princeton Review. Rice is also rated as a best value among private universities by Kiplinger’s Personal Finance.