David Ruth
713-348-6327
david@rice.edu
Jade Boyd
713-348-6778
jadeboyd@rice.edu
Nanoscale drawbridges open path to color displays
Rice develops first method for reversible color changes with metal nanoparticles
HOUSTON — (Dec. 4, 2015) — A new method for building “drawbridges” between metal nanoparticles may allow electronics makers to build full-color displays using light-scattering nanoparticles that are similar to the gold materials that medieval artisans used to create red stained-glass.
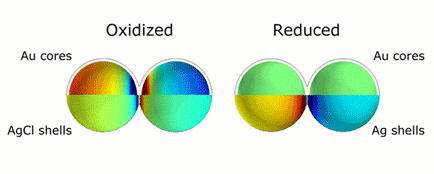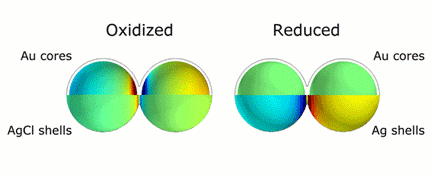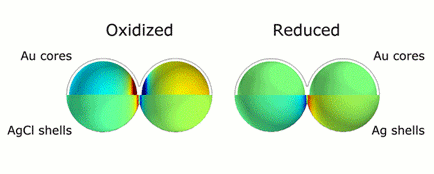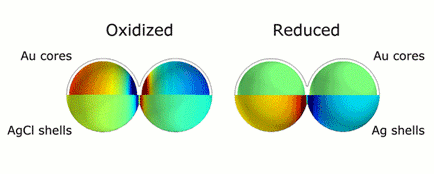
This animation illustrates the markedly different colors of light that are scattered thanks to plasmonic shifts that occur when no metal bridges are present (left) and when they are (right). Credit: H. Zhang/Rice University
“Wouldn’t it be interesting if we could create stained-glass windows that changed colors at the flip of a switch?” said Christy Landes, associate professor of chemistry at Rice and the lead researcher on a new study about the drawbridge method that appears this week in the open-access journal Science Advances.
The research by Landes and other experts at Rice University’s Smalley-Curl Institute could allow engineers to use standard electrical switching techniques to construct color displays from pairs of nanoparticles that scatter different colors of light.
For centuries, stained-glass makers have tapped the light-scattering properties of tiny gold nanoparticles to produce glass with rich red tones. Similar types of materials could increasingly find use in modern electronics as manufacturers work to make smaller, faster and more energy-efficient components that operate at optical frequencies.
Though metal nanoparticles scatter bright light, researchers have found it difficult to coax them to produce dramatically different colors, Landes said.
Rice’s new drawbridge method for color switching incorporates metal nanoparticles that absorb light energy and convert it into plasmons, waves of electrons that flow like a fluid across a particle’s surface. Each plasmon scatters and absorbs a characteristic frequency of light, and even minor changes in the wave-like sloshing of a plasmon shift that frequency. The greater the change in plasmonic frequency, the greater the difference between the colors observed.
“Engineers hoping to make a display from optically active nanoparticles need to be able to switch the color,” Landes said. “That type of switching has proven very difficult to achieve with nanoparticles. People have achieved moderate success using various plasmon-coupling schemes in particle assemblies. What we’ve shown though is variation of the coupling mechanism itself, which can be used to produce huge color changes both rapidly and reversibly.”
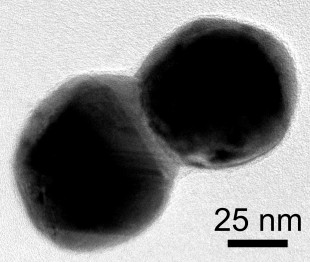
This electron microscope image shows a dimer of silver plated gold nanoparticles. A layer of silver connects the particles. Credit: D. Swearer/Rice University
To demonstrate the method, Landes and study lead author Chad Byers, a graduate student in her lab, anchored pairs of gold nanoparticles to a glass surface covered with indium tin oxide (ITO), the same conductor that’s used in many smartphone screens. By sealing the particles in a chamber filled with a saltwater electrolyte and a silver electrode, Byers and Landes were able form a device with a complete circuit. They then showed they could apply a small voltage to the ITO to electroplate silver onto the surface of the gold particles. In that process, the particles were first coated with a thin layer of silver chloride. By later applying a negative voltage, the researchers caused a conductive silver “drawbridge” to form. Reversing the voltage caused the bridge to withdraw.
“The great thing about these chemical bridges is that we can create and eliminate them simply by applying or reversing a voltage,” Landes said. “This is the first method yet demonstrated to produce dramatic, reversible color changes for devices built from light-activated nanoparticles.”
Byers said his research into the plasmonic behavior of gold dimers began about two years ago.
“We were pursuing the idea that we could make significant changes in optical properties of individual particles simply by altering charge density,” he said. “Theory predicts that colors can be changed just by adding or removing electrons, and we wanted to see if we could do that reversibly, simply by turning a voltage on or off.”
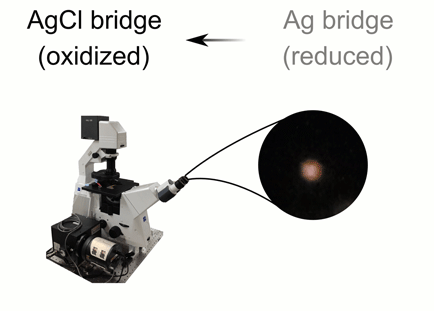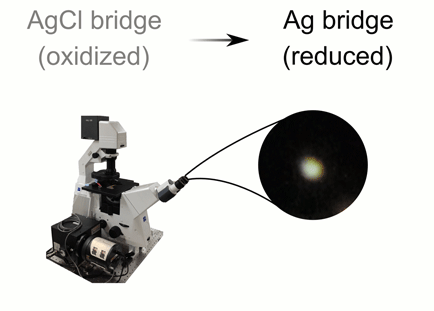
This animation illustrates the green and orange hues of light that are scattered thanks to plasmonic shifts that occur when metal bridges are present (bottom) and when they are not (top). Credit: C. Byers/Rice University
The experiments worked. The color shift was observed and reversible, but the change in the color was minute.
“It wasn’t going to get anybody excited about any sort of switchable display applications,” Landes said.
But she and Byers also noticed that their results differed from the theoretical predictions.
Landes said that was because the predictions were based upon using an inert electrode made of a metal like palladium that isn’t subject to oxidation. But silver is not inert. It reacts easily with oxygen in air or water to form a coat of unsightly silver oxide. This oxidizing layer can also form from silver chloride, and Landes said that is what was occurring when the silver counter electrode was used in Byers’ first experiments.
“It was an imperfection that was throwing off our results, but rather than run away from it, we decided to use it to our advantage,” Landes said.
Rice plasmonics pioneer and study co-author Naomi Halas, director of the Smalley-Curl Institute, said the new research shows how plasmonic components could be used to produce electronically switchable color-displays.

Rice researchers (clockwise from left) Da Huang, Stephan Link, Christy Landes, Anneli Hoggard, Hui Zhang, Wei-Shun Chang, Chad Byers and Ben Hoener are part of a team that developed the first method for producing dramatic, reversible color changes from light-activated nanoparticles. Photo by Jeff Fitlow/Rice University
“Gold nanoparticles are particularly attractive for display purposes,” said Halas, Rice’s Stanley C. Moore Professor of Electrical and Computer Engineering and professor of chemistry, bioengineering, physics and astronomy, and materials science and nanoengineering. “Depending upon their shape, they can produce a variety of specific colors. They are also extremely stable, and even though gold is expensive, very little is needed to produce an extremely bright color.”
In designing, testing and analyzing the follow-up experiments on dimers, Landes and Byers engaged with a brain trust of Rice plasmonics experts that included Halas, physicist and engineer Peter Nordlander, chemist Stephan Link, materials scientist Emilie Ringe and their students, as well as Paul Mulvaney of the University of Melbourne in Australia.
Together, the team confirmed the composition and spacing of the dimers and showed how metal drawbridges could be used to induce large color shifts based on voltage inputs.
Nordlander and Hui Zhang, the two theorists in the group, examined the device’s “plasmonic coupling,” the interacting dance that plasmons engage in when they are in close contact. For instance, plasmonic dimers are known to act as light-activated capacitors, and prior research has shown that connecting dimers with nanowire bridges brings about a new state of resonance known as a “charge-transfer plasmon,” which has its own distinct optical signature.

Dayne Swearer and Emilie Ringe analyzed materials from the drawbridge experiments using Rice's microscopy facility, which now includes a Titan Themis, one of the nation's most powerful electron microscopes. Photo by Jeff Fitlow/Rice University.
“The electrochemical bridging of the interparticle gap enables a fully reversible transition between two plasmonic coupling regimes, one capacitive and the other conductive,” Nordlander said. “The shift between these regimes is evident from the dynamic evolution of the charge transfer plasmon.”
Halas said the method provides plasmonic researchers with a valuable tool for precisely controlling the gaps between dimers and other multiparticle plasmonic configurations.
“In an applied sense, gap control is important for the development of active plasmonic devices like switches and modulators, but it is also an important tool for basic scientists who are conducting curiosity-driven research in the emerging field of quantum plasmonics.”
Additional study co-authors from Rice include Dayne Swearer, Mustafa Yorulmaz, Benjamin Hoener, Da Huang, Anneli Hoggard and Wei-Shun Chang.
The research was supported by the National Science Foundation, the Welch Foundation, the American Chemical Society, the Air Force Office of Scientific Research and the Smalley-Curl Institute.
-30-
High-resolution IMAGES are available for download at:
https://news2.rice.edu/files/2015/12/1204-REV-charge2.gif
CAPTION: This animation illustrates the markedly different colors of light that are scattered thanks to plasmonic shifts that occur when no metal bridges are present (left) and when they are (right).
CREDIT: H. Zhang/Rice University
https://news2.rice.edu/files/2015/12/1204-REV-group-lg.jpg
CAPTION: Rice researchers (clockwise from left) Da Huang, Stephan Link, Christy Landes, Anneli Hoggard, Hui Zhang, Wei-Shun Chang, Chad Byers and Ben Hoener are part of a team that developed the first method for producing dramatic, reversible color changes from light-activated nanoparticles.
CREDIT: Jeff Fitlow/Rice University
https://news2.rice.edu/files/2015/12/1204-REV-dimer-lg.jpg
CAPTION: This electron microscope image shows a dimer of silver plated gold nanoparticles. A layer of silver connects the particles.
CREDIT: D. Swearer/Rice University
https://news2.rice.edu/files/2015/12/1204-REV-clcb-lg.jpg
CAPTION: Christy Landes and Chad Byers
CREDIT: Jeff Fitlow/Rice University
https://news2.rice.edu/files/2015/12/1204-REV-micro.gif
CAPTION: This animation illustrates the green and orange hues of light that are scattered thanks to plasmonic shifts that occur when metal bridges are present (bottom) and when they are not (top).
CREDIT: C. Byers/Rice University
https://news2.rice.edu/files/2015/12/1204-REV-titan-lg.jpg
CAPTION: Dayne Swearer and Emilie Ringe analyzed materials from the drawbridge experiments using Rice’s microscopy facility, which now includes a Titan Themis, one of the nation’s most powerful electron microscopes.
CREDIT: Jeff Fitlow/Rice University
The DOI of the Science Advances paper is:
10.1126/sciadv.1500988
A copy of the paper is available at:
http://advances.sciencemag.org/content/1/11/e1500988
Related photonic research from Rice:
Rice researchers make ultrasensitive conductivity measurements — June 10, 2015
https://news2.rice.edu/2015/06/10/rice-researchers-make-ultrasensitive-conductivity-measurements-2/
‘Squid skin’ metamaterials project yields vivid color display — Sept. 15, 2014
https://news2.rice.edu/2014/09/15/squid-skin-metamaterials-project-yields-vivid-color-display/
Rice lab uses CMOS-compatible aluminum for on-chip color detection — Aug. 25, 2014
https://news2.rice.edu/2014/08/25/biomimetic-photodetector-sees-in-color-2/
Rice unveils method for tailoring optical processors — May 21, 2013
https://news2.rice.edu/2013/05/21/rice-unveils-method-for-tailoring-optical-processors/
Rice uses light to remotely trigger biochemical reactions — Dec. 13, 2012
https://news2.rice.edu/2012/12/13/rice-uses-light-to-remotely-trigger-biochemical-reactions/
Rice unveils super-efficient solar-energy technology — Nov. 19, 2012
https://news2.rice.edu/2012/11/19/rice-unveils-super-efficient-solar-energy-technology/
Located on a 300-acre forested campus in Houston, Rice University is consistently ranked among the nation’s top 20 universities by U.S. News & World Report. Rice has highly respected schools of Architecture, Business, Continuing Studies, Engineering, Humanities, Music, Natural Sciences and Social Sciences and is home to the Baker Institute for Public Policy. With 3,910 undergraduates and 2,809 graduate students, Rice’s undergraduate student-to-faculty ratio is 6-to-1. Its residential college system builds close-knit communities and lifelong friendships, just one reason why Rice is ranked No. 1 for best quality of life and for lots of race/class interaction by the Princeton Review. Rice is also rated as a best value among private universities by Kiplinger’s Personal Finance. To read “What they’re saying about Rice,” go to http://tinyurl.com/AboutRiceUniversity.


