Rice-led experiments demonstrate solid-state carbon nanotube ‘templates’
A simple way to turn carbon nanotubes into valuable graphene nanoribbons may be to grind them, according to research led by Rice University.
The trick, said Rice materials scientist Pulickel Ajayan, is to mix two types of chemically modified nanotubes. When they come into contact during grinding, they react and unzip, a process that until now has depended largely on reactions in harsh chemical solutions.
The research by Ajayan and his international collaborators appears in Nature Communications.
To be clear, Ajayan said, the new process is still a chemical reaction that depends on molecules purposely attached to the nanotubes, a process called functionalization. The most interesting part to the researchers is that a process as simple as grinding could deliver strong chemical coupling between solid nanostructures and produce novel forms of nanostructured products with specific properties.

Rice graduate student Mohamad Kabbani grinds nanotubes with a mortar and pestle. A chemical reaction takes place as the altered nanotubes are forced together, unzipping them into graphene nanoribbons. Photo by Jeff Fitlow
“Chemical reactions can easily be done in solutions, but this work is entirely solid state,” he said. “Our question is this: If we can use nanotubes as templates, functionalize them and get reactions under the right conditions, what kinds of things can we make with a large number of possible nanostructures and chemical functional groups?”
The process should enable many new chemical reactions and products, said Mohamad Kabbani, a graduate student at Rice and lead author of the paper. “Using different functionalities in different nanoscale systems could revolutionize nanomaterials development,” he said.
Highly conductive graphene nanoribbons, thousands of times smaller than a human hair, are finding their way into the marketplace in composite materials. The nanoribbons boost the materials’ electronic properties and/or strength.
“Controlling such structures by mechano-chemical transformation will be the key to find new applications,” said co-author Thalappil Pradeep, a professor of chemistry at the Indian Institute of Technology Chennai. “Soft chemistry of this kind can happen in many conditions, contributing to better understanding of materials processing.”
In their tests, the researchers prepared two batches of multi-walled carbon nanotubes, one with carboxyl groups and the other with hydroxyl groups attached. When ground together for up to 20 minutes with a mortar and pestle, the chemical additives reacted with each other, triggering the nanotubes to unzip into nanoribbons, with water as a byproduct.
“That serendipitous observation will lead to further systematic studies of nanotubes reactions in solid state, including ab-initio theoretical models and simulations,” Ajayan said. “This is exciting.”
The experiments were duplicated by participating labs at Rice, at the Indian Institute of Technology and at the Lebanese American University in Beirut. They were performed in standard lab conditions as well as in a vacuum, outside in the open air and at variable humidity, temperatures, times and seasons.
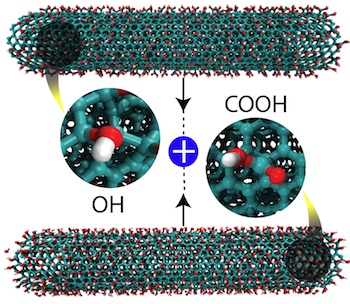
Researchers led by materials scientists at Rice University discovered that altering carbon nanotubes with carboxyl (COOH) and hydroxyl (OH) groups and grinding them together produces nanoribbons. The find could lead to novel nanostructured products with specific properties. Click the image for a larger version. Courtesy of Mohamad Kabbani
The researchers who carried out the collaboration on three continents still don’t know precisely what’s happening at the nanoscale. “It is an exothermic reaction, so the energy’s enough to break up the nanotubes into ribbons, but the details of the dynamics are difficult to monitor,” Kabbani said. “There’s no way we can grind two nanotubes in a microscope and watch it happen. Not yet, anyway.”
But the results speak for themselves.
“I don’t know why people haven’t explored this idea, that you can control reactions by supporting the reactants on nanostructures,” Ajayan said. “What we’ve done is very crude, but it’s a beginning and a lot of work can follow along these lines.”
Co-authors are Rice graduate students Chandra Sekhar Tiwary, Sehmus Ozden and Yongji Gong; Pedro Autreto, Gustavo Brunetto and Professor Douglas Galvao of the State University of Campinas, Brazil; Anirban Som and K.R. Krishnadas of the Indian Institute of Technology Madras; Robert Vajtai, a senior faculty fellow at Rice, and Ahmad Kabbani, an adjunct faculty member at Rice and a professor of chemistry at the Lebanese American University, Beirut.
The research was supported by the Air Force Office of Scientific Research and its Multidisciplinary University Research Initiative; the Brazilian National Council for Scientific and Technological Development, CAPES (Coordination of Improvement of Higher Education Personnel) and the São Paulo Research Foundation; the Center for Computational Engineering and Sciences at the State University of Campinas, and the Nano Mission, Government of India.


If my thinking is right, a dehydration reaction is happening here. if i am not ambitious, can this mechanochemistry be used to break a benzene molecule or a non-degradable substance. That will be more interesting.