David Ruth
713-348-6327
david@rice.edu
Mike Williams
713-348-6728
mikewilliams@rice.edu
Proteins’ passing phases revealed
Rice U. theorists combine structural data, genomic analysis to predict short-lived conformations of proteins
HOUSTON – (Dec. 5, 2013) – A new method to identify previously hidden details about the structures of proteins may speed the process of novel drug design, according to scientists at Rice University.
A unique combination of computational techniques and experimental data helped Rice theorists predict intermediate configurations of proteins that, until now, have been hard to detect.
The work should be significant for pharmaceutical companies that design drugs through painstaking processes and at great cost by eliminating some of the trial and error in identifying new sites on proteins that could be more easily manipulated to treat disease, said Rice biological physicist José Onuchic.
The research appears online this week in an open-access paper in the Proceedings of the National Academy of Sciences.
Onuchic and his team integrated its direct coupling analysis (DCA) method based on genomic databases with structure-based models (SBM) of proteins to produce simulations of how proteins progress through different functional states. “It has been long known that this information is encoded in the protein sequences, but it has been hard to extract,” said Faruck Morcos, a postdoctoral researcher at Rice and lead author of the paper.
Because they’ve been conserved by evolution, it’s likely these intermediate states have important functions, Onuchic said.
Proteins, the engines that drive biological processes, usually collapse into their native states in the blink of an eye. X-ray crystallography and, more recently, nuclear magnetic resonance spectroscopy are the most common tools to see how the amino acids in a protein chain arrange themselves based on their attractive and repulsive energies, but they say nothing about the forms the proteins may take along the way, Onuchic said.
He said the methods are “fine for small proteins or enzymes that have a single functional structure. But large proteins like molecular motors or signaling proteins have multiple functional conformations, some of them too short-lived to be captured by X-ray crystallography. The problem has been that we have lots of information about the sequences and not enough about the structures.”
Onuchic and his colleagues at the Center for Theoretical Biological Physics, based at Rice’s BioScience Research Collaborative, are working to fix that. They employ DCA to compare and predict direct structural contacts between amino acids (called residues) from the proteins’ genomic roots. Protein sequences are built by ribosomes from genetic data conveyed by messenger RNA molecules. DCA also allows researchers to compare genetic data across protein families and determine which residues in those families co-evolved. This information guides the physics-based simulation toward functional conformations that have been conserved through evolution.
Simulations at Rice that combined DCA and structural data revealed competing residue contacts that were unique to configurations of proteins with multiple conformations and led to the discovery of intermediate states, Onuchic said. The researchers focused on glutamate-receptor and ligand-binding proteins that go through large conformational changes, like opening and closing, upon binding. They serve as sensors for chemical signals and fulfill their tasks by changing their configurations to trap chemical compounds, Morcos said.
With the hybrid SBM+DCA program and improved imaging methods in development, theorists and experimentalists will be able to compute and then confirm the process by which specific proteins go about their business, Onuchic said.
“You can’t design drugs in a vacuum,” he said. “These simulations give us possible targets to subject to much more detailed simulations. Supercomputers will be important tools for that.
“Think of a simple simulation as like looking at the ground from 10,000 feet. When you see towns you’re interested in, you can go back and do a detailed probe of each one of them. In the same way, we find conformations in the protein’s landscape we think are important and that we can return to for a more detailed look.”
Co-authors are Biman Jana, a Rice postdoctoral researcher, and Terence Hwa, a professor in the Department of Physics at the University of California, San Diego. Onuchic is Rice’s Harry C. and Olga K. Wiess Professor of Physics and Astronomy.
The research was supported by the Welch Foundation, the National Science Foundation and the Cancer Prevention and Research Institute of Texas. The researchers utilized the Data Analysis and Visualization Cyberinfrastructure (DAVinCI) supercomputer supported by the National Science Foundation and administered by Rice’s Ken Kennedy Institute for Information Technology.
-30-
Read the open-access paper at http://www.pnas.org/content/early/2013/11/27/1315625110.full.pdf+html
This news release can be found online at https://news2.rice.edu/2013/12/05/proteins-passing-phases-revealed/
Follow Rice News and Media Relations via Twitter @RiceUNews
Related Materials:
Center for Theoretical Biological Physics: http://ctbp.rice.edu
José Onuchic bio: http://chemistry.rice.edu/FacultyDetail.aspx?p=51BE2F2C673C5991
Images and animated gifs for download:
Animated gif:
https://news2.rice.edu/files/2013/12/Movie-1a.gif
YouTube:
A simulation produced by the SBM+DCA method shows an L-leucine binding protein in the process of opening and closing. The program created at Rice University uses genomic data and structural information about proteins to predict intermediate folding stages thought to have important functions. (Credit: Onuchic Lab/Rice University)
Animated gif:
https://news2.rice.edu/files/2013/12/Movie-2.gif
YouTube:
A simulation produced via SBM+DCA shows the open, closed and an intermediate twisted stage in a D-ribose binding protein. The program created at Rice University uses genomic data and structural information about proteins to predict intermediate folding stages thought to have important functions. (Credit: Onuchic Lab/Rice University)
https://news2.rice.edu/files/2013/12/1209_PROTEINS-1-web.jpg
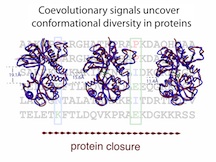
A new method by scientists at Rice University has the power to predict topologies in proteins that form after folding, when the proteins are performing their functions. The red and blue lines are closely matching structures of a protein predicted by the SBM+DCA program (blue), which combines co-evolutionary information from sequences and data from X-ray crystallography from one conformation (red/left) to predict other functional conformations (red/center and right). The Rice method could help reduce trial and error, as well as cost, in the development of new drugs by predicting previously potential and previously unknown binding sites. (Credit: Faruck Morcos and Biman Jana/Rice University)
Located on a 300-acre forested campus in Houston, Rice University is consistently ranked among the nation’s top 20 universities by U.S. News & World Report. Rice has highly respected schools of Architecture, Business, Continuing Studies, Engineering, Humanities, Music, Natural Sciences and Social Sciences and is home to the Baker Institute for Public Policy. With 3,708 undergraduates and 2,374 graduate students, Rice’s undergraduate student-to-faculty ratio is 6-to-1. Its residential college system builds close-knit communities and lifelong friendships, just one reason why Rice has been ranked No. 1 for best quality of life multiple times by the Princeton Review and No. 2 for “best value” among private universities by Kiplinger’s Personal Finance. To read “What they’re saying about Rice,” go to http://tinyurl.com/AboutRiceU.
If you do not wish to receive news releases from Rice University, reply to this email and write “unsubscribe” in the subject line. Office of News and Media Relations – MS 300, Rice University, 6100 Main St., Houston, TX 77005