Jeff Falk
713-348-6775
jfalk@rice.edu
Mike Williams
713-348-6728
mikewilliams@rice.edu
Mismatched materials can be tough enough
Rice University scientists analyze molecular detail of cement-polymer bonds
HOUSTON – (June 10, 2013) – Rice University researchers have for the first time detailed the molecular mechanism that makes a particular combination of cement and polymer glue so tough.
The theoretical research by Rice materials scientist Rouzbeh Shahsavari and his group led to a fine picture of how hydrogen bonds control the properties of hybrid organic-inorganic materials. The finding has implications for understanding the interface bonding that is often a roadblock to improved composite properties.
The research is detailed in the American Chemical Society journal Langmuir.
The Rice researchers said their work has the potential to help fine-tune advanced materials well beyond the cement-polymer compound they studied. “Natural materials like bones, teeth and shells all have a hybrid of soft and stiff material inside, arranged in a brick-and-mortar pattern,” Shahsavari said. “This has inspired humans to build engineered composites by mimicking those natural designs.”
Building such composites requires a clear understanding of what’s happening when materials that aren’t necessarily compatible are combined.
“This could have applications for composites in cars, airplanes and civil engineering materials,” Shahsavari said. “Here, our focus is on inorganic silicates like cement (a key strengthening component of concrete) interacting with a polymer to provide a hybrid composite with high toughness and ductility. Otherwise, cement by itself is a brittle material.”
He said understanding bonds at the molecular level should help manufacturers design stronger, lighter composites from the bottom up. For example, cars that use advanced compounds could be lighter and more fuel-efficient while retaining the toughness that lets them absorb energy in a crash by crumpling instead of shattering. The same analogy is true for civil engineering composites that absorb the energy of an earthquake rather than fracture and collapse.
“Toughness by definition is the ability of the material to deform before fracture,” he said.
Shahsavari, graduate student and lead author Navid Sakhavand and former Rice postdoctoral researcher Prakash Muthuramalingam started by studying recent experiments on polymer-silicate compounds (polyvinyl alcohol mixed with cement in gel form) that suggested hydrogen bonds must be responsible for interfacial adhesion. They determined that very different geometries across the interface of the two materials complicate the process of figuring out the hydrogen-bonding pattern and how the molecules might bond and/or tear under stress.
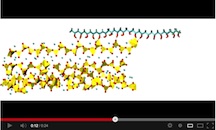
But it wasn’t impossible. The Rice researchers built molecular models that combined strands of polyvinyl alcohol and layered chains of tobermorite, a mineral they said is a natural analog of the previously studied cement gel.
At the molecular interface, they determined the hydrogen bonds most responsible for adhesion don’t quite line up. Subjecting their computer models to shear forces showed them how hydrogen bonds on both sides would break and reform as the polymer was pulled across the silicate. Subsequent calculations based on the energies of individual atomic pairs let them determine the seven types of hydrogen bonds possible where the two materials meet, and precisely which type of hydrogen bonds and how many of them should line up for maximum toughness.
This might be a bit counterintuitive, Shahsavari said, since a hydrogen bond is typically weak, but the cooperative action of several can result in significant adhesion and toughness. This can help determine the optimum overlap length and design parameters for manufacturing tough hybrid composites.
“The overall goal is to improve mechanical properties,” Sakhavand said. “We started with concrete, which is the most-used composite material on Earth. Billions of dollars are spent on it every year just in the United States. Any small improvement we can make for concrete is going to significantly change the industry.”
The theoretical models they’ve developed can be applied in many ways, Sakhavand said.
“All of these can easily be extended to any synthetic material,” he said, suggesting their formula can help experimentalists cut the amount of time spent on trial and error in the design of novel compounds.
The research team performed calculations on the Data Analysis and Visualization Cyber Infrastructure (DAVinCI) system funded by the National Science Foundation and operated by the Ken Kennedy Institute for Information Technology at Rice. An IBM Shared University Research Award in partnership with Cisco, QLogic and Adaptive Computing also supported the research.
-30-
See an animation of the molecular model here:
Read the abstract at http://pubs.acs.org/doi/abs/10.1021/la4014015
Follow Rice News and Media Relations via Twitter @RiceUNews
Related Materials:
Multiscale Materials Modeling Lab: http://rouzbeh.rice.edu/default.aspx
Image for download:
https://news2.rice.edu/files/2013/06/0610_TOUGH-1-web.jpg
Rice University researchers Rouzbeh Shahsavari, left, and Navid Sakhavand analyzed the molecular interface between cement and a polymer in a new Langmuir paper. (Credit: Jeff Fitlow/Rice University)